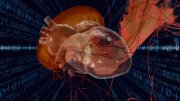
When Kevin Kit Parker took his daughter to the New England Aquarium years ago, he wasn’t thinking about soft robotics or artificial hearts. He was simply a father, watching his child delight in a jellyfish pulsing silently through the water.
“We saw the jellyfish swimming around,” he recalls. “And I said, I’m going to build that—because it looks like a heart.”
That moment sparked a decade-long journey, inspiring Parker, the Tarr family professor of bioengineering and applied physics at the Harvard Paulson School of Engineering and Applied Sciences (SEAS), to apply the rhythms of jellyfish to the mechanics of the human heart. His Disease Biophysics Group works on developing what Parker calls “biohybrid robots”: machines powered by living cardiac (or heart) muscle cells.
These robots are created by mimicking the bodies of jellyfish, stingrays, and manta rays through a process called “reverse tissue engineering.” Stem cells from hearts are layered onto soft robotic scaffolds made from natural and synthetic polymers. “Biologists don’t have the same design tools engineers do,” Parker says. “So, we make them. We had to learn how to use muscle cells as a building material.” He adds, “You end up doing materials science, tissue engineering, and physiology all at once.”
In doing so, Parker aims to uncover the fundamental laws of muscular pumps—laws which he believes may hold the key to understanding heart disease, the world’s leading cause of death. “Maybe the reason we can’t cure certain forms of heart disease,” he says, “is because we don’t understand those basic laws. When they’re violated, that’s when disease occurs.”
For Parker, marine life offers a natural model. “Most marine animals, with the exception of crustaceans, are designed to pump, just like your heart,” he explains. “By building fish, jellyfish, and stingrays, we have learned a lot about the heart itself.”
To test their designs, Parker and his students compare their robotic constructs to real jellyfish. “I’m not going to give [students] a human neonatal heart for a control experiment,” he says, referring to a heart of a newborn child. “But I can send a graduate student on a bicycle with a Ziploc bag to the aquarium to get a jellyfish.”
Ultimately, Parker—who also co-directs the Center for Advancing Therapeutic Discovery at Boston Children’s Hospital—has the goal of building an artificial heart to replace malformed hearts in pediatric patients.
But for the time being, Parker is using these marine-inspired designs to help lead efforts to create a “cardiovascular biodigital twin.” If realized, this “twin” will be a virtual model of the human heart that can simulate how it functions, responds to stress, and reacts to therapies.
In 2022, Parker joined a collaboration called the “Bio Digital Twin (BioDT) Initiative,” which includes researchers from NTT Research’s Medical and Health Informatics (MEI) Lab in Silicon Valley, the National Cerebral and Cardiovascular Center (NCVC) in Osaka, Japan, and the Technical University of Munich (TUM). On November 4 and 5, collaborators from the BioDT Initiative came to SEAS for a summit to discuss their progress. Panelists included Joe Alexander, the director of MEI Lab; Bernhard Wolfrum, a professor of neuroelectronics at the TUM School of Computation; Keita Saku, the laboratory chief at NCVC; and Parker.
As Parker and the researchers discussed, a working cardiovascular biodigital twin could transform medicine. Over time, this biodigital twin model, running on a computer in an intensive care unit, could evolve into patient-specific replicas capable of predicting how an individual’s heart might respond to medication or treatment. Using data from patients, lab-grown heart tissue, and animal studies, the goal is to train the digital model to mimic the mechanical and electrical behavior of a real heart, including its elasticity, contraction strength, and blood flow.
This digital replica could enable predictions based on “what if” clinical scenarios, serving as a live reference from which to guide treatment—from determining whether surgery is necessary to fine-tuning devices like pacemakers or extracorporeal membrane oxygenation (ECMO) systems, which use manmade hearts to temporarily support the body when a person's own organs are unable to do so.
For example, in cases of acute conditions, such as myocardial infarction or acute heart failure, doctors could use the twin to (within seconds) forecast how different drugs or treatment combinations might affect cardiac function. As the technology evolves, it could also extend to chronic cardiac conditions, which tend to develop over time due to remodeling of the heart and blood vessels. The biodigital twin could integrate continuous data streams like blood pressure, heart rate variability, fluid balance, and biochemical markers to model these gradual changes. This would allow the twin to simulate disease progression over weeks or months and predict how lifestyle, medications, or comorbidities influence long-term cardiac health.
Parker’s three-dimensional, marine-inspired models are directly informing the designs of these digital twins, providing critical biophysical data that allows for the heart’s mechanical and electrical characteristics to be represented virtually.
This concept, if realized, could reduce strain on cardiologists—or even, according to Alexander, who led a large part of the conversation at SEAS, allow primary care physicians to act as cardiologists, especially in understaffed and overburdened hospitals, or in remote or impoverished parts of the world. The twins could also help provide remote diagnostics for patients from afar, enabling at-home monitoring to predict heart failure before symptoms appear. Finally, they could revolutionize drug development, allowing therapies to be tested on virtual organs long before human trials.
If these ideas all sound futuristic, they are…but not by much. Twenty years ago, Parker was studying the basic mechanics of heart tissue. Today, he’s part of a team helping build a digital version of the organ.
The work remains rooted in the same curiosity that piqued Parker’s interest at the aquarium. “Nature had already solved the problem of how to move fluid through soft tissue,” he says. “All we had to do was pay attention.”