Elsie Sunderland grew up where the sea was as present as the land: a small rural community in Nova Scotia, Canada. It’s an area steeped in natural beauty and intimately tied to the ocean. But Sunderland, Cabot associate professor of environmental science and engineering, also remembers an early lesson in the fragility of that connection; in her teenage years, the community suffered financially as the natural resource its economy relied on—fishing—failed when Atlantic cod stocks collapsed.
Humans have long seen oceans as sources of endless bounty. “We thought that there were so many fish in the ocean that we could never possibly impact the populations,” Sunderland says. Again and again, that assumption has proven false; two-thirds of the world’s fisheries are considered overexploited.
“People struggle with this idea that we could impact planetary level processes,” she says. “But we do. There’s irrefutable evidence on many, many scales that we’re changing the natural functioning of these ecosystems.”
Such planetary-scale changes are not easy to study or understand. It has taken considerable effort spanning decades, for instance, for researchers around the world to collect the data and to assemble the complex computer models that allow other people to comprehend how human greenhouse-gas emissions are changing the Earth’s climate system. Sunderland’s research is focused on different, but equally complex, questions: how are human societies transforming the environment, particularly the oceans, through the pollutants they release—and how do those transformations boomerang to affect human health?
Humans have also treated the ocean as an endless repository for anything disposable—sewage or trash or chemical byproducts of industry—but mounting evidence suggests there are limits: from coastal “dead zones” caused by polluted runoff, to enormous pools of plastic trash collecting in the oceans. Coal-fired power plants alone release more than 200 hazardous pollutants into the atmosphere—many of which eventually end up in the ocean—and hundreds of other substances pour each day into rivers and oceans as part of urban runoff, or with effluent from industry and wastewater-treatment plants. There’s a huge gap in knowledge about what Sunderland calls the “global chemical experiment” that civilization is conducting with these substances: Where do they go, and how do they make their way into wildlife and humans?
Her lab has approached this question by focusing on a few compounds of concern to human health, particularly methylmercury and man-made chemicals called PFAS (per- and polyfluoroalkyl substances) that are used in such consumer products as food packaging, nonstick products, and stain-repellent fabrics. The lab connects research on chemistry, environmental dynamics, ecology, and epidemiology by piecing together a large-scale picture of how these compounds wend their way through the environment—and ultimately depicts the oceans as part of a closed, interconnected system that can be mapped and understood.
The Grasshopper Effect
Sunderland’s upbringing taught her about the fragility of ocean ecosystems, but it also gave her a sense of optimism about action. During her teens and early adulthood, she watched her community’s environmental crisis turn into an awakening. As the local government looked for other industries to develop, a toxic-waste incineration plant was proposed. Sunderland’s father launched a community action group to fight the proposal, and she wrote op-eds, participated in protests and public hearings, and helped research the health and environmental effects of toxic-waste pollution, stirring her interest in chemistry and toxicology. The opposition movement blossomed into a growing environmental awareness. Sunderland assisted the municipal government in a project to launch the first large-scale composting and recycling facility in North America, which kept about 90 percent of the local municipal waste from a landfill.
The experience inspired her to work in policy, and after getting her Ph.D. in environmental toxicology, in 2003 she took a job with the U.S. Environmental Protection Agency, then the most powerful such agency in the world. She spent five years there, at one point developing a rule to regulate air pollution from coal-fired utilities. But ultimately she decided she could make a more enduring contribution through academic research.
At Harvard, Sunderland’s focus has been mercury pollution, a prime example of how the waste products people put into the oceans come back to harm human health. Mercury is a naturally occurring heavy metal that can be toxic to the nervous system of humans and other animals. Elemental mercury is most familiar to people in its liquid form, called quicksilver, but it also exists as a gas, and can interact with other chemicals to form organic and inorganic compounds. Most of the earth’s mercury stores are buried deep within the planet’s crust, but some is released to the environment naturally through processes such as volcanic activity, including through hydrothermal vents on the ocean floor. Humans have also been extracting and using mercury since antiquity; it’s been used to separate silver and gold from raw ore, and is present in many products, from batteries to light bulbs and paints.
Mercury is still used in mining. Small-scale, artisanal gold extraction, an informal industry practiced by an estimated 10 million to 19 million people in 70 countries, has become the biggest source of emissions today—one that causes grave pollution problems for local communities and ecosystems. Coal-fired power plants are another major source of oceanic mercury pollution. Annually, humanity burns several billion tons of coal. Thus, even though coal contains only trace amounts of mercury, the sheer volume combusted by humans—when combined with the metal’s unique properties—has created a global problem.
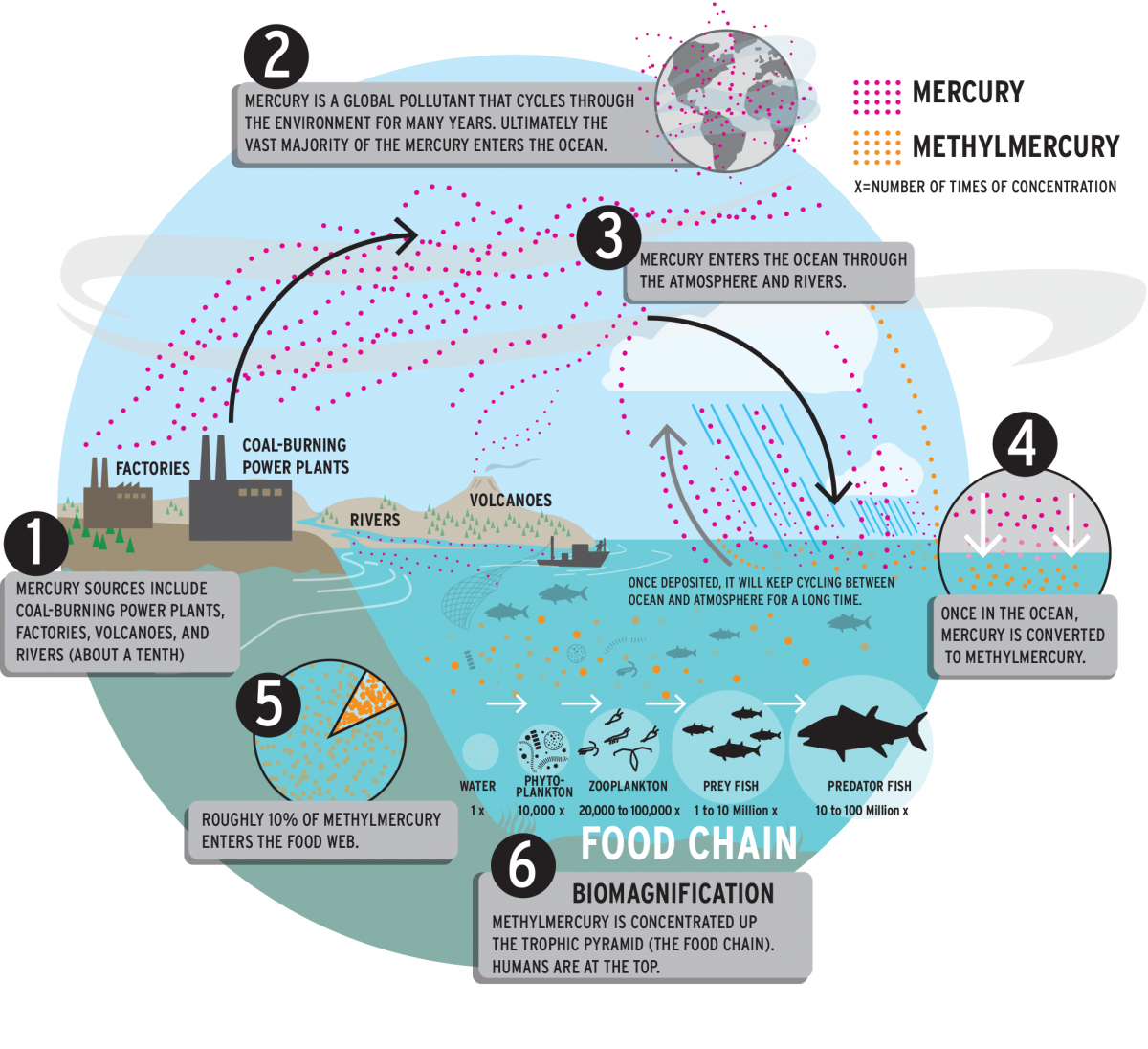
THE MERCURY CYCLE
Mercury traces a map across the planet that is determined by its particular chemistry. When it is released from power plants, some binds with water and dust particles in the atmosphere, causing it to be deposited on nearby land and water. But most of the released mercury is “so stable in the atmosphere, it can travel long distances and be mixed at least hemispherically, if not globally,” Sunderland says.
When it eventually falls to earth, microorganisms can convert it into an organic form, called methylmercury, which accumulates in living organisms. In some environments, methylmercury can undergo further reactions that re-release it into the atmosphere as elemental mercury again. This phenomenon, called the “grasshopper effect,” means that mercury keeps cycling through water, land, and air for long periods of time.
To build biogeochemical models—mathematical representations that capture the larger scope and dynamics of global mercury pollution’s cycles and effects on humans—Sunderland’s research connects disparate information, including inventories of mercury emissions, knowledge of environmental chemistry, and direct measurements of the metal in the environment. Because mercury is a naturally occurring element, she says, many people view methylmercury exposure in humans as a “natural” phenomenon. But her work shows otherwise. “The cumulative releases of mercury from human sources since 1850 are 78 times larger than natural emissions from the same time period,” she says. Like carbon dioxide, mercury may be a “natural” substance, but at such high concentrations it should be considered a human-caused pollution; she estimates that 88 percent of the mercury in the ocean derives from human sources.
Mercury’s tenacity means the biogeochemical models must take into account cumulative emissions over time. David Streets, an energy and environmental policy scientist at Argonne National Laboratory, has collaborated with Sunderland’s lab on a cumulative accounting of mercury emissions; they’ve also shown how mercury emissions have changed over time. “What’s changed are the sources and the regional distribution,” he says. At one time, mercury came from mining booms in the Americas, and later, coal combustion in the United States and Europe. Today, coal combustion is higher in Asia and Africa, even as many developed countries have cut back on coal use and mercury-containing products.
About half of the mercury released into the atmosphere makes its way into the oceans, often in sediments at or near coastlines. Such long-term marine burial is the best chance for mercury to be removed from its perpetual cycles. But Sunderland points out that, even there, human activity can interfere: dredging and trawling may release mercury from the ocean floor, a phenomenon her group is beginning to study.
Methylmercury and Seafood: The Minamata Incident
Direct contact with mercury has long been known to cause poisoning (the use of mercury in felt-making was associated with “Mad Hatter’s disease,” a potentially fatal neurological condition, beginning in the seventeenth century). But it was not until the mid twentieth century that exposure to methylmercury from fish and shellfish consumption raised health concerns. In the 1950s, methylmercury poisoning, now called Minamata disease, was first recognized when residents in Minamata City, Japan, fell gravely ill after eating fish and shellfish contaminated with mercury from a chemical plant. Methylmercury is now understood to be toxic to nerve cells; at high exposures, it can cause nerve tingling, muscle weakness, sensory loss, paralysis, and even death. But research on the epidemiology and toxicology of methylmercury has found more subtle effects at low doses, too. “Over the years, the evidence has been extended, and we now believe adverse effects can be seen within the range [to which] Americans are normally exposed,” says adjunct professor of environmental health Phillipe Grandjean, who has studied methylmercury’s human impacts and collaborates with Sunderland.
The greatest effect occurs on the developing nervous systems of fetuses, infants, and children. When researchers look at large groups of babies and children, they see lower average scores in cognitive ability, motor skills, and memory in those exposed to high levels of methylmercury. When researchers look at large groups of babies and children, they see lower average scores in cognitive ability, motor skills, and memory in those exposed to high levels of methylmercury. The children are “still within the normal range,” Grandjean says, “but are not quite functioning as well as they should have.”
Fish and seafood consumption remains by far the primary route through which people are exposed to mercury. Biological tissue is a potent storehouse for methylmercury; the mercury build-up in one organism is passed along to any other organism that eats it, and on up the food chain. This process, called “biomagnification,” means that mercury emitted into the ocean can quickly and potently come back to haunt consumers. Compared to its presence in seawater, methylmercury concentrates by a factor of a million or more in a large tuna or swordfish.
What’s not always appreciated, Sunderland says, is how the methylmercury aggregating in the open oceans touches U.S. consumers. The seafood market is sprawling and global; people living in New England and other coastal areas may assume that local fisheries play a large role in their diet, but in reality most Americans are eating fish caught in faraway open-ocean fisheries. Sunderland’s team led a recent study, published in Environmental Health Perspectives, which calculated how different categories of seafood from different regions of the world contributed to Americans’ mercury exposure from 2000 to 2010. The biggest source of methylmercury, Sunderland’s team found, is fish caught in the Pacific Ocean. And the largest source is tuna, which is enduringly popular in the United States and accounts for 37 percent of domestic methylmercury exposure.
Health authorities have struggled to craft public-service messages about seafood intake. The United States and several other countries have sought to limit exposure to methylmercury in pregnant women, infants, and children, by issuing guidelines that specify weekly portion sizes and suggest limits to consumption of certain high-mercury species.
The problem? Fish and seafood contain nutrients such as omega-3 fatty acids that are also known to be highly beneficial for development. And replacing a portion of fish with something that is low in mercury but less nutritious isn’t a good strategy, either. “It’s not a simple message,” Sunderland acknowledges, and recommendations can quickly become confusing.
“From a nutritional perspective,” she says, “humans would ideally consume more fish, not less.” Wealthier populations can respond to declining fish stocks and pollution by finding healthy substitutes. But many people around the world depend on fish and seafood for daily subsistence (see “The Double Smack of Fishery Collapse”). Globally, she suggests, the best solution is to stop pollution at its source, rather than expect individuals to follow complicated guidelines, “because everybody can agree that we want less methylmercury in our fish.”
The Minamata Convention, signed by 128 countries in 2013 and put into effect in 2017, is a first step toward global action on mercury pollution, addressing issues such as the mining, transport, storage, and disposal of mercury, and its use in products. Sunderland’s teams have estimated that global mercury emissions declined by 30 percent between 1990 and 2010, mostly because of a phase-out of mercury in commercial products, declining coal combustion in many developed countries, and the use of technologies that capture pollutants released by coal combustion.
These results show that policies can have measurable impacts, though it may take decades to significantly reduce the total amount of mercury pollution, because of its centuries-long lifetime in the environment. But the Trump administration’s recent effort to roll back the Clean Power Plan, which set carbon emissions limits on power plants, heads in the opposite direction. Discussions about reducing fossil-fuel energy and greenhouse gases often take place separately from discussions about pollution, Sunderland says, but “They’re very much related, because many energy sources like coal release hundreds of hazardous air pollutants at the same time.”
Chemical Whack-a-Mole
Thousands of different chemicals now being used in industry and commerce are also entering the environment in growing concentrations. Unlike mercury, their environmental and biological behavior is often unknown.
The PFAS chemical class that Sunderland has been studying is wholly a human invention, widely used to give consumer products coatings that make them waterproof, stain-resistant, or non-stick, and also used in fire-fighting foams. Although PFAS come in many forms, they all contain fluorine atoms that attach extremely stably to chains of carbon atoms.
The same properties that make these chemicals repel oil, water, and heat for long periods of time also make them extremely persistent in the environment. Within the entire PFAS family, “There are about 3,000 different compounds that we know of, and in that 3,000, hundreds have been detected in the environment,” she says. They are also readily detectable in wildlife and in humans. The U.S. Centers for Disease Control’s National Health and Nutrition Examination Survey found PFAS in 98 percent of blood samples from thousands of Americans collected in 2003 and 2004.
These substances make their way into wastewater, rivers, and the oceans. Some react with other chemicals and degrade into different forms, Sunderland says, but eventually “They reach this very stable form and then they just sit there and are transported by water movement.” In animals, PFAS can affect reproduction, immunity, and liver and kidney function. Philippe Grandjean has found that they may interfere with the effectiveness of childhood vaccines: infants who have high PFAS levels later have a lower antibody response to vaccines, suggesting that the substances depress the immune system. His research also finds that the chemicals are passed along through breast milk, and accumulate in babies who breastfeed (see “Mercury on the Brain,” May-June 2004, page 12). He and Sunderland are collaborating with the University of Rhode Island on a new research center funded by the National Institute of Environmental Health Sciences to study PFAS in the environment and their effects, particularly through drinking water.
PFAS were listed as persistent organic pollutants under the Stockholm Convention in 2009. The most well-studied of these compounds, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS), were voluntarily phased out of production in the United States and most other countries because of concerns about their environmental and health effects. Many companies have been replacing those substances with different types of PFAS that have shorter chains of carbon molecules, making them less likely to accumulate in biological tissue.
But the impact isn’t clear. “The chemical manufacturing industry shifted to these shorter carbon-chained alternatives, which we know very little about,” Sunderland explains. Even as her lab collects data on certain compounds, they are being phased out in favor of new “custom” molecules with unknown properties. Researchers, she says, are “just chasing the chemistry, trying to figure out what’s being released into the environment.” It’s a problem that Joseph Allen, assistant professor of exposure assessment sciences at the Harvard T.H. Chan School of Public Health, has called a game of chemical “whack-a-mole.”
Sunderland hopes to shed light on general properties of chemicals that persist in the environment, to help guide decisions about which ones are safer to manufacture. Ultimately, she believes, a different approach to developing and using industrial chemicals is needed. “Obviously, the chemical manufacturing industry is essential for modern society. But we could do it a lot better than we do,” she says. Compounds that are known to cause harm should be replaced, but there should be more emphasis on knowing the “end of pipe” impacts of those replacements before they’re manufactured. “When you’re thinking about mitigating toxicity, you want to do it before any products have been developed,” she says. In the past, the environmental impacts of chemicals have been seen as a concern separate from their development; the educational curriculum for chemical engineers, she points out, doesn’t typically require learning about environmental science or toxicology.
Facing vast pollution problems like these can easily make people feel defeated. “We need to propose solutions and think about [these issues] constructively,” says Sunderland. Many of those solutions will require changes in national and international policies, education, and industry regulations. But some changes will begin locally. Sunderland points to the hundreds of communities near military bases affected by PFAS contamination from firefighting foams, many of which are beginning to come forward and draw attention to the problem. As she has learned from her own upbringing, when communities begin to care about environmental issues close to home, big and unexpected changes can emerge.










