In 2007, associate professor of medicine Samia Mora and colleagues published a study of exercise that sought to understand why physical activity is salutary. They already knew that exercise reduces the risk of cardiovascular disease as much as cholesterol-lowering statin drugs do. By analyzing biomarkers in the blood of 27,055 women participating in a long-term study, and other objective measures, they hoped to tease out the source of this effect. How much of the benefit was attributable to improved blood pressure? To lower body weight? Or to something else? The women had donated blood in the 1990s when they entered the study. Eleven years later, the researchers analyzed this frozen blood to see if they could find anything that correlated with long-term cardiovascular outcomes such as heart attack and stroke. “We were actually surprised that reduced inflammation was the biggest explainer, the biggest contributor to the benefit of activity,” says Mora, “because we hadn’t hypothesized that. We knew that regular exercise does reduce inflammation over the long term, but we also knew that acute exercise transiently increases inflammatory biomarkers during and immediately after exertion.” About a third of the benefit of regular exercise, they found, is attributable to reduced inflammation.
The anti-inflammatory effect of exercise was much greater than most people had expected. That raised another question: whether inflammation might also play a dominant role in other lifestyle illnesses that have been linked to cardiovascular disease, such as diabetes and dementia.
In 2017, two cardiologists at Brigham and Women’s Hospital in Boston, who suspected such a link, published the results of a human clinical trial that will forever change the way people think about inflammation. The trial, which involved more than 10,000 patients in 39 countries, was primarily designed to determine whether an anti-inflammatory drug, by itself, could lower rates of cardiovascular disease in a large population, without simultaneously lowering levels of cholesterol, as statin drugs do. The answer was yes. But the researchers went a step further, building into the trial additional tests seeking to clarify what effect the same anti-inflammatory drug, canakinumab, might have on illnesses seemingly unrelated to cardiovascular disease: arthritis, gout, and cancer. Only the researchers themselves, and their scientific colleagues, were unsurprised by the outcome. Lung cancer mortality dropped by as much as 77 percent. Reports of arthritis and gout also fell significantly.
In medicine, believing something is true is not the same as being able to prove it. Because the idea that inflammation—constant, low-level, immune-system activation —could be at the root of many noncommunicable diseases is a startling claim, it requires extraordinary proof. Can seemingly unconnected illnesses of the brain, the vasculature, lungs, liver, and joints really share a deep biological link? Evidence has been mounting that these common chronic conditions—including Alzheimer’s, cancer, arthritis, asthma, gout, psoriasis, anemia, Parkinson’s disease, multiple sclerosis, diabetes, and depression among them—are indeed triggered by low-grade, long-term inflammation. But it took that large-scale human clinical trial to dispel any lingering doubt: the immune system’s inflammatory response is killing people by degrees.
Now the pertinent question is why, and what can be done about it. The pharmaceutical industry is deeply interested in finding ways to stop inflammation with medicines like canakinumab, an orphan drug that blocks a specific pro-inflammatory pathway called IL-1beta. But some researchers suggest that the inflammatory process—a normal and necessary part of the natural immune response—has itself has been misunderstood. Scientists know that the process can be turned on and off, but have only recently understood that this doesn’t mean normal physiology will resume once the inflammation caused by infection, injury, or irritant has been shut down. Instead, the restoration of health is an active phase of the inflammatory process itself, facilitated by a little-known class of molecules called pro-resolving mediators—the protectins, resolvins, maresins, and lipoxins—brimming with marvelous, untapped, regenerative capacities.
Origins of Atherosclerosis
The 2017 clinical trial, called CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study), is the result of a long-term collaboration between Paul Ridker and Peter Libby, who suspected as long ago as the 1980s that inflammation played a role in cardiovascular disease. Ridker, an epidemiologist who is Braunwald professor of medicine, came to this conclusion through studies of cardiac patients. He is the physician-scientist who first demonstrated that a molecule called C-reactive protein (CRP), easily measured by a simple and now ubiquitous blood test, could be used like a thermometer to take the temperature of a patient’s inflammation. Elevated CRP, he discovered years ago, predicts future cardiovascular events, including heart attacks. Although nobody knows what it does biologically, this marker is downstream from IL-1beta, and thus provides a reliable yardstick of that pro-inflammatory pathway’s level of activation.

Paul Ridker and Peter Libby
Photograph by Jim Harrison
Libby, the Mallinckrodt professor of medicine, is a bench scientist and clinician with expertise in the study of heart disease. In the 1980s, orthodoxy within the cardiovascular establishment held that circulating fats (including cholesterol) build up in the arteries of patients with progressive cardiovascular disease. But no one knew why or how the plaques formed. It took work by some of the most distinguished cardiology researchers of the era to lay the groundwork that eventually produced an understanding of the molecular mechanisms that drive deposition of those plaques.
Today, in his Harvard Medical School (HMS) office, Libby sketches the origins of atherosclerosis. The interior walls of blood vessels, he explains, are made from smooth muscle cells, lined in turn with endothelial cells that are in direct contact with circulating blood. When a problem arises, caused by anything from cholesterol to bacteria, the vascular system recruits white blood cells, the immune system’s front-line guardians, to the site. Two Harvard professors of pathology, Michael Gimbrone Jr. and the late Ramzi Cotran, figured out that naturally occurring adhesion molecules could attract these white blood cells and get them to stick to the endothelium lining the arteries. Their experiments implicated a pro-inflammatory signal called interleukin-1 (IL-1), which is produced by both circulating and tissue-based immune cells.
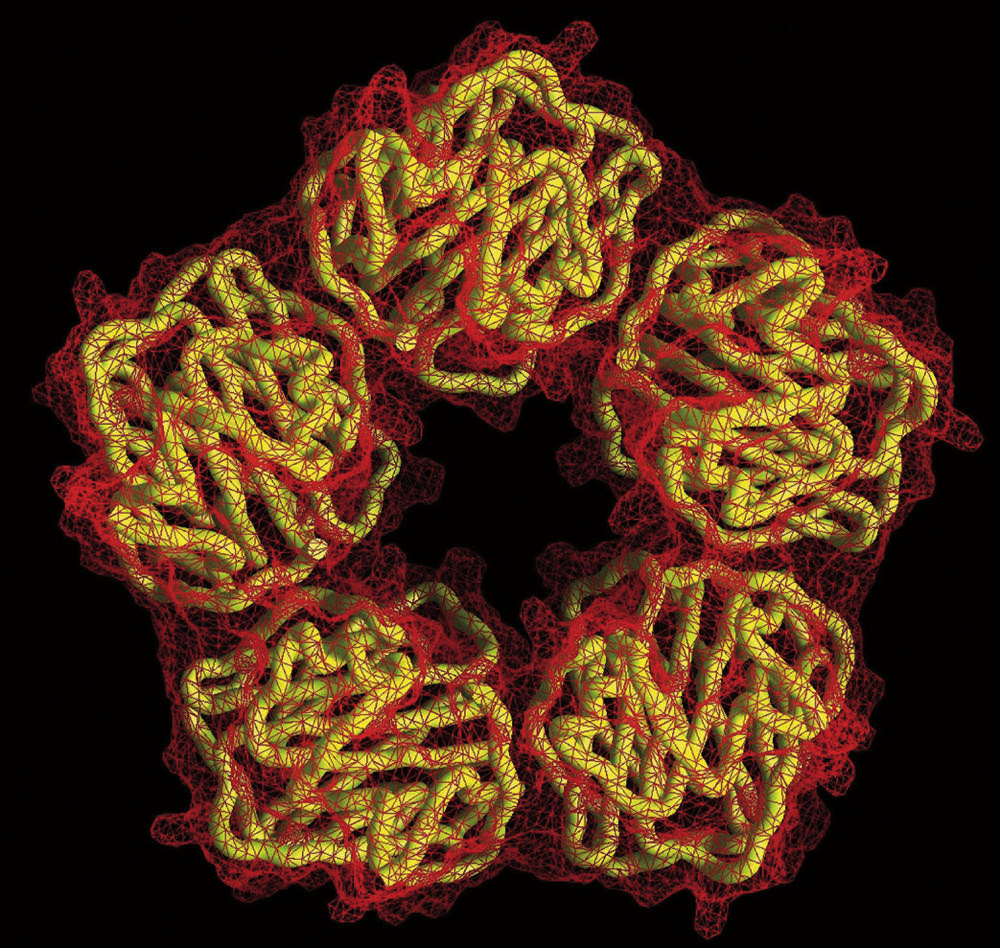 The pentameric molecular structure of C-reactive protein, a biomarker of inflammation that circulates in the blood. Cardiologists use CRP to predict a person’s future risk of heart attack.
The pentameric molecular structure of C-reactive protein, a biomarker of inflammation that circulates in the blood. Cardiologists use CRP to predict a person’s future risk of heart attack.Courtesy of T. Greenhough & A. Shrive/Wellcome Image
Libby, then at Tufts, followed their work closely. IL-1 had been discovered in 1977 by one of his Tufts colleagues, Charles Dinarello, who had been focused on understanding what causes fever, one of the cardinal signs of inflammation. The others, described by Aulus Cornelius Celsus in the first century c.e., are redness (rubor), which occurs when the endothelial lining of arteries dilates to permit more blood flow; swelling (tumor), caused by endothelial cells leaking protein, which carries water; and pain (dolor). By measuring the factors in rabbits’ blood, Dinarello was able to isolate and then clone the specific factor—called a pyrogen—that causes fever: interleukin-1.
But before this inflammatory pyrogen even had a name, Dinarello gave some to Libby, whose lab was down the hall. Libby discovered that arterial wall cells not only responded to IL-1, but could secrete it. This was heretical, he explains, because it was thought that only “a properly pedigreed immunological cell” could produce such signals. Now it was clear that the cells of the artery walls were capable of summoning an immune response. Libby further found that IL-1, by altering gene expression in local blood vessel cells, amplifies its signal at the site of the disease. But, “No one in cardiology was interested in inflammation” then, Libby notes. In fact, none of his early work on IL-1 appears in the cardiology literature: “My papers were rejected,” he recalls; “my grants turned down.” In 1986, he published his first paper showing that the lining of the arteries could produce IL-1 in the American Journal of Pathology.
The Evolution of Excessive Inflammation
Today, inflammation is a focus of intense research in many fields. Roni Nowarski, assistant professor of neurology and immunology, explains that inflammation is important across a range of seemingly distinct pathologies because immune cells are everywhere, even resident in organs, where they play an important role in monitoring and maintaining health. The paradigm that everyone knows—that the immune system’s front line consists of circulating white blood cells that patrol the body to guard against infection and injury—is a bit misleading. An important arm of the immune system resides outside the blood vessels. Pac-Man-like macrophages occupy tissues, where they engulf and digest invading pathogens, debris, and dying cells. An invaluable role of these tissue macrophages is to “act as sensors,” Nowarski says. “They have hard-wired mechanisms to detect signals that are out of the ordinary,” and so play a critical role in maintaining a healthy equilibrium. “If there is any fluctuation,” he notes, “the role of these cells is to return the system to this point of homeostasis.”
These tissue-based white blood cells can also call for backup. When that happens, the heavy guns of the immune system, neutrophils, are first to arrive on the scene. These are “potent, aggressive cells” that can kill infectious agents, Nowarski explains, but “can also cause a lot of damage to healthy tissue.” That’s why most neutrophils are short-lived, with a tightly regulated lifespan of just a few hours: unchecked, they would cause serious harm. Neutrophils, which originate in bone marrow, also play a role in relaxing the endothelial barrier that separates blood from tissue, so immune cells can cross that barrier to reach the site of attack. Other signals—like the IL-1beta protein that Libby and Ridker blocked in their trial—promote adhesion, in order to capture circulating immune cells that reach the damaged tissue. This stickiness, though desirable in the short term, is also the basis of the process that can lead to atherosclerosis if it continues indefinitely. The endgame of a healthy immune response, on the other hand, involves cleanup, says Nowarski: even the death and uptake of neutrophils as they are gobbled by macrophages serve as signals to resolve inflammation.
Why inflammation sometimes doesn’t resolve, and becomes chronic instead, is in some sense easily explained in evolutionary terms. “If I’m living 70,000 years ago at a time of food shortage,” says Ridker, “and there’s a drought, the 5 to 10 percent of people who will survive that drought are likely to have insulin resistance”—a tendency to store more calories as fat. “They’re going to live a little longer,” he continues. “When it finally rains, food comes, and that’s the survivor group. In a modern world of too much food, [insulin resistance] leads to diabetes. But in prehistory, it’s terribly important for survival.” While stored fat is beneficial during times of famine, it also harbors potentially damaging pro-inflammatory signaling molecules (see “Eating to Excess: Metabolic Inflammation,” below).
A second evolved factor is that prior to the development of antibiotics, disease “wiped out half the population before age five. So, people were under evolutionary pressure to have a hyperactive immune system.” Now, “most everybody survives childhood infections,” thanks in large part to vaccines. “But this hyperactive immune system remains, and adversely affects aging.”
“The third piece—beyond starvation and infection—is trauma,” Ridker says. “The saber-toothed tiger—or for women, bleeding to death during childbirth—selects on a genetic basis for hypercoagulable blood. So here we are, by definition all of us lucky enough to be alive today, with a consistent ancestry all the way back to the beginning. And we have all inherited a pro-inflammatory, insulin resistant, pro-coagulable state. Under the circumstances,” he continues, the fact that “we have an epidemic of diabetes and heart disease makes complete sense.” Evolutionary pressures have shaped a physiological system which is phenomenally well suited for surviving childhood infection, starvation, and predation. “But it contributes to many disorders of chronic aging, because from an evolutionary perspective, if you’ve had your kids, you’re kind of done.”
Evolution also explains why the underlying biology appears so similar from one disease to the next. Kennedy professor of child neurology and mental retardation Rudy Tanzi studies Alzheimer’s disease, which is well known for causing plaques and tangles in the brain. In 2008, Tanzi and his colleagues discovered a gene that clearly played a role in the development of the disease, but didn’t know how it worked. Five years later, they discovered that the gene is the “on” switch for inflammation in the brain. Tanzi says the work that has most influenced his thinking is the body of resilience studies conducted by Teresa Gomez-Isla, an associate professor of neurology based at Massachusetts General Hospital, who has shown that “you can have a brain full of plaques and tangles, but if you don’t have neuroinflammation, you don’t get the disease”—a finding that neatly parallels the pathological interactions between plaques and inflammation in atherosclerosis. This basic research on inflammation thus underlies understanding of entire classes of human illness.
Such similarities among diseases of metabolism and inflammation suggest that they are indeed rooted far back in evolutionary history. Gökhan S. Hotamisligil, Simmons professor of genetics and metabolism at the Harvard T.H. Chan School of Public Health, has constructed his research for the past 25 years on the premise that this shared history suggests shared biological mechanisms as well. The ailments that cluster most dramatically, he points out, are “chronic metabolic diseases” such as diabetes, cardiovascular disease, stroke, Alzheimer’s, nerve degeneration, and cancer. In general, a person who has any one of these illnesses is more likely to develop the others. “This is exactly the same cluster that emerges during aging,” he says. “All of the age-related pathologies exhibit as clusters of non-communicable diseases, except in a much shorter period of time, and earlier in the lifespan.
“That tells us that these illnesses are part of our natural history, our biological heritage,” continues Hotamisligil, who directs the Sabri Ülker Center for Metabolic Research. “If these two clusters emerge in two separate conditions in exactly the same way, that must come from deeply embedded evolutionary vulnerabilities.”
“Chronic inflammation is uniformly damaging and is absolutely causal to the process, because if you interfere with it, you can reverse the pathology.”
Critics might suggest that inflammation is just a symptom in these diseases, rather than a cause. But Hotamisligil says, unequivocally, “Chronic inflammation is uniformly damaging and is absolutely causal to the process, because if you interfere with it, you can reverse the pathology.” And this ability to control such diseases simply by reversing inflammation is a biological response, dating far back to the time of a common ancestor, that has been retained across diverse species of animals to the present day, he says, pointing to experimental evidence: “If you can make Drosophila [fruit fly] diabetic, and then block the inflammatory response systems, you can cure diabetes in Drosophila, the same way you can reverse it in the mouse, in primates, and in humans, provided that you do it with the right tools. Of course, the higher the organism, the more complex these pathways are, so it takes more effort to define the precise mechanisms to manipulate.”
Eating to Excess: Metabolic Inflammation
Seeking the origins of inflammation, Hotamisligil asks, “What is the most primordial process in the emergence of life? Metabolism. Energy management. And then, of course, the next critical process to emerge within cells is the ability to defend. And so that brings in the immune system, which is younger than metabolism, but much older than many other systems.” In some of the simplest organisms, he points out, energy management and immune functions are packaged in the same organ, such as the fruit fly’s fat body. But even as these functions became specialized and divided among three or four organs in more complex organisms, they retained their evolutionary memory. “They share many tricks,” he says, because the “immune response is enormously expensive, energy-wise.” The intimate relationship between the metabolic and immune systems, he believes, has been maintained because it takes “tremendous energy to mount an effective immune response. While this link is essential to maintain health and homeostasis, abnormalities that develop over time, as in the case of obesity, carry a great risk of damage.”
The metabolic stress that is a hallmark of modern life, the stress that the body has not evolved to handle, is constant eating, he continues. When people eat, energy and nutrients enter the body rapidly, are processed, produce in turn a lot of by-products, and then need to be reduced to “functional substances that are distributed throughout the body, and then disappear very quickly. Many cells and tissues actually undergo a huge amount of stress during this process,” he explains, “as they store appropriate nutrients and dispose of harmful intermediates.” Part of this process also involves mounting an immune response. “The pancreas, for example, must secrete four to five hundred milliliters of enzymes every day” to be able to manage the incoming energy load with every meal. “If you place these organs under constant stress, they start malfunctioning.” The consequence is that “right now, one out of every 10 individuals has diabetes. One out of every four individuals has fatty liver disease. And if you reach a certain age, one out of every three individuals will develop neurodegenerative disease.”
The metabolic stress that underlies these conditions comes from the daily imbalance between how much energy people consume and how much they need, and can process in a healthy manner. The long-term consequence of overconsumption, combined with lack of sufficient expenditure, is stored energy—the accumulation of fat. Excess body fat, especially in the wrong places, is an additional risk factor for inflammation.
Clinical studies implicating stored fat as a source of inflammation have been buttressed by basic research that shows that adipose tissue—body fat—is laced with immune cells, which become more abundant with weight gain, perhaps because fat cells can secrete alarm signals that summon white blood cells. “A fat cell is almost like a primitive immune cell,” says Hotamisligil. “It can request the assistance of immune cells when in trouble, but if the stress continues, and the immune cells remain, they start changing their character and behavior from helpful to harmful.”
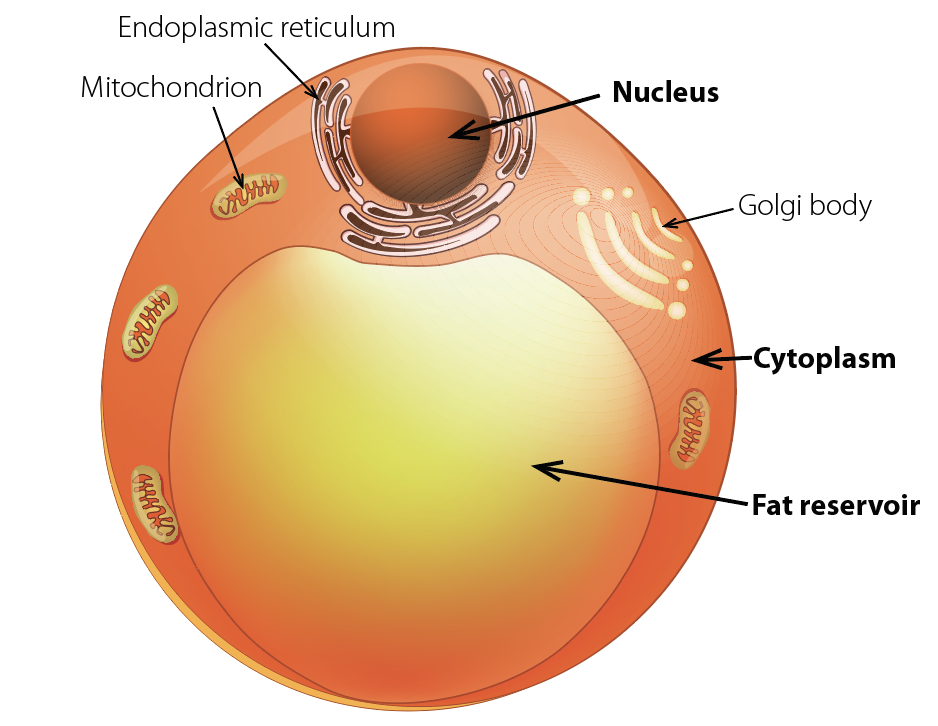
A fat cell, or adipocyte, is more than 90 percent triglyceride. If the thin cell wall enclosing this droplet of fat ruptures, an inflammatory response ensues.
iStock Images
And there is additional evidence that the physical structure of fat cells, or adipocytes, which have been described by Stephen O’Rahilly of the University of Cambridge as resembling a fried egg atop a beach ball of fat—with a tiny rim of cytoplasm encircling the droplet of triglyceride—puts them at risk of rupture. Adipocytes are “cells at the edge under the best of circumstances,” explains Hotamisligil. Ninety percent or more of a fat cell’s volume is triglyceride, a substance chemically similar to diesel fuel. The remaining 10 percent is all the space it has for the organelles that perform the normal functions of cell biology. This bloated cell is thus vulnerable to stress, even to the point of bursting and death. When overloaded with stored lipid, fat cells begin to lose their functional and structural integrity and may start spilling their toxic cargo. When cells fail like this, the immune system kicks in, initially to assist in clean-up. Macrophages engorge themselves on the leaking fuel, and may die themselves during this process. But in the long run, what is meant to be a mutually beneficial interaction between the metabolic and immune systems turns into a very dangerous and harmful relationship. Obese individuals thus live in a state of chronic stress and inflammation; in fact, many people do, because their energy intake vastly exceeds their needs. Hotamisligil calls this chronic energy overload, and the resulting abnormal immune response, metaflammation: metabolic inflammation.
“It is pretty clear that inflammation is a bad actor in obesity,” says Korsmeyer professor of cell biology and medicine Bruce Spiegelman. His lab was the first to establish the mechanism linking obesity to inflammation—in 1993, when he and Hotamisligil, then a doctoral student, discovered that fat cells produce an inflammatory signal that interferes with the body’s ability to regulate blood sugar. This, in turn, increases the risk of developing Type 2 diabetes.
But inflammation in muscle, he says, is “much more complicated.” In fact, “it is likely that you require inflammation in exercise,” and that the inflammatory response should not be suppressed—for example, by taking ibuprofen—because that signal is likely “telling the muscle to remodel.”
Together with Rasmussen professor of immunohematology Diane Mathis, Spiegelman is about to begin studying exercised muscle, a natural system that incorporates and manages inflammation on a regular basis. Mathis studies regulatory T-cells (Tregs), white blood cells that are actively involved in maintaining the internal stability (homeostasis) of tissues. Recently, she has sought to understand the role of Tregs in repairing muscle injured as a consequence of physical trauma or disease.
Now, by studying muscle that has been exercised, she and Spiegelman hope to gain a better understanding of how inflammation is “supposed to happen, and how it eventually resolves.” The sequence, he says, occurs in four steps: “There’s damage, inflammation, resolution of inflammation, and repair. These phases are very different.” He and Mathis hope to learn how these processes unfold—and ultimately, perhaps, to learn how to stimulate, support, or mimic the body’s natural mechanisms of resolution and repair.
Controlling Inflammation, Boosting the Immune System
Epidemiological studies have helped clarify the importance of lifestyle choices in controlling inflammation. Samia Mora’s 2007 research highlighted the role of exercise. In 2018, she and her colleagues published a study of the Mediterranean diet, which is known to improve cardiovascular health (and also thought to protect against neurodegenerative diseases such as Alzheimer’s and Parkinson’s). Mora examined the effect of this diet on the women who previously participated in the exercise research, 20 years after they had entered the original study. They found that adherence to a diet rich in vegetables, fruit, nuts, legumes, and olive oil, that also includes fish and chicken, but that is very low in red and processed meat and sugary foods or drinks, led to a lower risk of adverse cardiovascular events. As in the exercise study, they found that about a third of the benefit was due to reductions in inflammation.

Samia Mora
Photograph by Jim Harrison
But some of the diet’s benefit could not be explained, meaning that an untested factor was enhancing its healthful effect. Mora speculates that the diet (which includes probiotic foods such as Greek yogurt) might support the health of the gut microbiome, or might stimulate the parasympathetic nervous system, as exercise does, to help people relax. Alternatively, the diet might be protective against oxidative stress of the kind that comes from pollution or smoking. Perhaps unsurprisingly, each of these possibilities is linked to inflammation.
The great difficulty with interventions involving altered diet and increased exercise is that these healthy habits aren’t aligned with preferences evolved during millennia of food scarcity. People already know what they should be doing—but for most, that knowledge doesn’t change behavior. Humans are hard-wired to conserve energy (see “Born to Rest,” September-October 2016, page 9), for example, and to prefer foods that are fatty, salty, and sugary.
This suggests that pharmaceutical interventions that block inflammation may be necessary to check the global epidemic of non-communicable disease. How to do that is not obvious, however. The intervention that Ridker and Libby devised, blocking a single inflammatory pathway with a drug, helped only a fraction of patients with cardiovascular disease. That is probably because a system as important to sustaining life as immune response has evolved redundancies: block one pathway and another will take over.
What the CANTOS study did establish was a biological principle, says Ridker: “Anti-inflammatory and immunosuppressive are not the same thing.” Suppressing an immune pathway like IL-1beta could have led to reactivation of tuberculosis infections or complications of HIV. But it didn’t. There was a slight increase in the risk of viral and bacterial infections—but these were “run of the mill infections,” he says. “Had we known in advance, we could very easily have taken care of these with some very simple antibiotics.”
This kind of experiment is important, he continues, because it will change the way drugs like canakinumab, currently classified as an immunosuppressant, are categorized. That opens new areas to investigate, he says: “We can actually give high-risk patients this kind of anti-inflammatory without risking immunosuppression. And that’s incredibly useful information for my infectious-disease colleagues.”
Some researchers believe, however, that to prevent certain inflammatory diseases, such as rheumatoid arthritis or lupus, multiple inflammatory pathways will need to be blocked to have a large effect. And blocking several of these signaling mechanisms simultaneously, they say, will almost surely suppress the immune system, exposing people to potentially fatal infections.
Hence scientists’ interests in another possibility that doesn’t include any risk of immunosuppression: resolving inflammation through a recently discovered class of molecules called specialized pro-resolving mediators.
A Super Family of Resolving Molecules
Gelman professor of anaesthesia Charles Serhan has been studying how inflammation ends for 25 years. He realized early on that after the immune system’s soldiers have fought off an invader, the battlefield is littered with bodies: dead cells and scattered debris. The fact that infection has been defeated does not mean that the affected tissue will automatically, passively, return to normal function. There is another process at work, factors that clean up the mess, remove bodies, and repair systems so that the physiological balance within the tissue can be restored. Working with other scientists around the world, he discovered a new class of molecules that actively resolve inflammation. “It turns out that there’s a whole super family of these,” he explains, “and it’s their collapse,” which occurs naturally with aging, that leads to chronic, unresolved immune-system stimulation. “These specialized pro-resolving mediators [SPMs] have been shown in many animal models to reverse inflammation.”

Charles Serhan
Photograph by Jim Harrison
SPMs are unusual immune-signaling molecules, in the sense that they are fats (lipid-derived small molecules), not proteins. They can also mute pain. Their precursors—the substances the body needs in order to synthesize these potent resolving compounds—are the essential fatty acids, including the omega-3 fatty acids EPA and DHA, and arachidonic acid.
In experiments with animals deficient in SPMs, Serhan has shown that injecting SPMs amplifies the magnitude of the healing response, causing injuries to mend more quickly. He emphasizes that reversing inflammation in this way is not the same as blocking it from occurring in the first place. When inflammatory pathways are turned off, there is always a risk that the immune response will be compromised, and that infection will ensue. The SPMs instead work in concert with the immune response by stimulating macrophages “to clear dead cells, debris, and bacteria,” he says. “Then they bring the system back to homeostasis, and begin to push the buttons to signal tissue regeneration.” (They even stimulate the Tregs that Diane Mathis studies to produce an anti-inflammatory signal called IL-10.)
Think about how over-the-counter anti-inflammatories such as ibuprofen and acetaminophen work. They block a particular signal. But Serhan discovered that aspirin works differently (and in a multi-faceted way): rather than blocking inflammatory signals, it attenuates them. In addition, it has mild anti-coagulant properties that are beneficial in atherosclerosis. And perhaps most importantly, aspirin stimulates the production of at least two classes of health-promoting SPMs. In work published as this magazine went to press, Serhan and colleagues showed that aspirin stimulates the production of a distinct type of SPM that fights cancer tumors in mice, and another SPM that inhibits cancer tumor formation in the first place.
Serhan has recently developed a method for creating blood profiles of individuals that reveal whether they have sufficient levels of these circulating resolving molecules, which include classes of compounds called resolvins, protectins, maresins, and lipoxins. Resolvins, for example, have proven beneficial against periodontitis in rabbits and retinopathy and colitis in mice. Protectins have proven effective in preventing ischemic stroke in rats, and against Alzheimer’s in humans. Lipoxins have attenuated pleurisy and cystic fibrosis in mice. And maresins have accelerated wound-healing in mice, and blocked the perception of pain.
More broadly, he has demonstrated the benefits of such SPMs in preventing neurodegeneration, and is beginning to study their use by professional football players, who suffer high rates of tissue injuries. Serhan has shown that SPMs can be used to control the inflammation that occurs when blood flow resumes to tissues that have been deprived of oxygen during surgery. And he has created an inflammation-resolving mouth rinse that has been tested in periodontal disease and shown to be safe. In earlier experiments, he demonstrated that pro-resolving eye drops can be used to control inflammation in the eye, which “naturally makes buckets of this stuff in tears, so you are bathing normally in pro-resolving mediators.” SPMs are also abundant in the brain, he has found. These are places where avoiding acute inflammation is absolutely critical: infections of the brain can be fatal, and in the eye can lead to blindness.
Colleagues of Serhan’s are using resolvins to control asthma and to stimulate surgical-wound healing. They are also investigating their effects on the microbiome. Earlier animal studies showed that resolvins reduce rheumatoid arthritis.
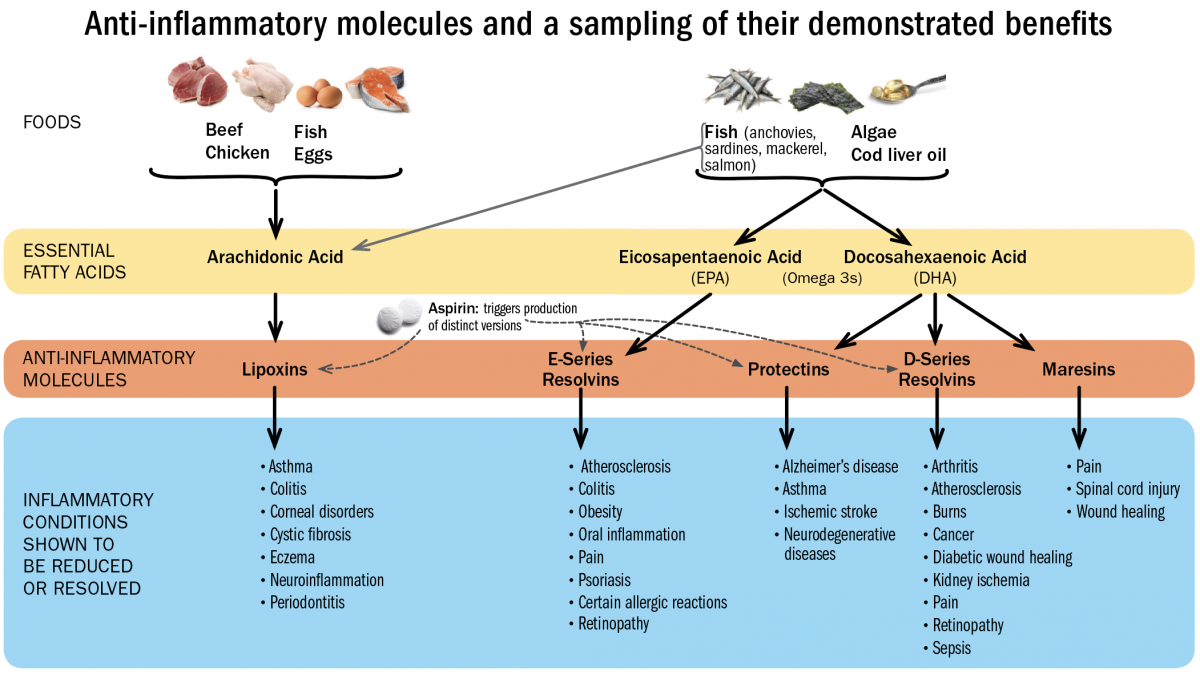
Because these compounds have not yet been synthesized as pharmaceuticals, maintaining healthy levels of SPMs is best supported by foods rich in the essential fatty acids EPA, DHA, and arachidonic acid. “There’s a reason they are called ‘essential,’” says Serhan. “You can only get them from your diet.” Fish contains all three, although arachidonic acid is also present in chicken, eggs, and beef, and EPA and DHA can be obtained from certain plant sources and algae. It’s ironic, he points out, that veterinary science has ensured that lab animals (and even pets) in the United States eat better than most people do, because animal food is fortified with omega-3s. Most Americans, he believes, don’t eat enough of them.
“To treat excessive inflammation...we don’t want to block the inflammatory response. We want to stimulate the resolution pathways.”
However illuminating Serhan’s molecular work has proven, though, the conceptual contribution of his research may be his lasting legacy. “One of things that we are trying to teach people from what we are learning is that we don’t want to knock anything out,” he says. “We want to fine-tune it.” Molecules such as IL-1beta—the target of the CANTOS trial—are important in the innate immune response, he explains. “To treat excessive inflammation, whether it is chronic or the result of an acute tissue injury, we don’t want to block the inflammatory response. We want to stimulate the resolution pathways.”
The scientific study of inflammation has transformed human understanding of this innate biological response. What once were considered merely symptoms—redness, swelling, fever, and pain—are now implicated as the source of many afflictions. For healing, Serhan foresees, we should also look within.








