For all the havoc and death caused by the coronavirus during the past 20 months, enough time has passed to reflect on a few significant issues. How will humanity cope with the rest of this pandemic—and prepare to respond better to the inevitable next one?
Much has been learned about SARS CoV-2, and more broadly, methods for combating a novel viral pathogen. Infectious-disease experts understand how the virus spreads across populations, and how contagious it has become: in the United Kingdom before vaccines were available, only national lockdowns managed to contain a fast-spreading variant. The U.K.’s experience presages disaster in the near future for low-income countries, whose access to vaccines remains limited even as more contagious variants proliferate.
Physician-scientists, using data gleaned from clinical trials, have painstakingly worked out which treatments are most effective at each stage of COVID-19’s complex progression. They no longer need to rely on guesswork to inform their decisions. Better, targeted treatments are in development.
Immunologists and virologists have developed safe and highly effective vaccines with extraordinary speed. Close study of these vaccines’ efficacy, and the complexity of the immune responses they elicit, has underscored the importance of clinical-trial data to assess their durability.
Many questions, of course, remain unanswered. Here, three scientists take stock of the virus’s epidemiology and pathogenesis, the diagnosis and treatment of the disease it causes, and the interplay of variants and vaccines—as well as the imperative of preparedness:
• Stryker and Johnston professor of global health Megan Murray knows the challenges of fighting infectious diseases in under-resourced settings, having closely observed the starkly different outcomes for countries like Rwanda, where a robust public-health infrastructure (and testing regime that put the United States’s to shame), kept the pandemic in check; and Peru, where infection rates have raged to levels that dwarf those of other nations, including India. Having studied tuberculosis for decades, Murray, director of research in the division of global health equity at Brigham and Women’s Hospital (BWH) and at Partners In Health, is well-acquainted with the global dynamics of respiratory diseases.
• Associate professor of medicine Lindsey Baden, a physician-scientist and director of clinical research in the division of infectious diseases at BWH, has been optimizing treatments for and clinical management of COVID-19 patients. He is also lead author of the first published SARS-CoV-2 vaccine clinical trial results, part of an ongoing study that is tracking the efficacy and durability of Moderna’s new mRNA vaccine technology over time.
• Castle professor of medicine and professor of immunology Dan Barouch, director of the center for virology and vaccine research at Beth Israel Deaconess Medical Center, helped develop the single-shot, nonprofit Johnson & Johnson/Janssen vaccine (see “Ending an Epidemic,” July-August 2020, page 18). He previously developed vaccines against HIV and Zika, and is actively involved in SARS-CoV-2 vaccine research and assessment.
Asymptomatic Spread
Murray, an epidemiologist, is an expert in the transmission dynamics of emerging infectious diseases—and a gifted communicator about the subject. As a regular guest on a weekly BBC program that answers questions about the pandemic, she prepares by reviewing the latest scientific literature. But her answer to most of the questions posed is still, “We don’t know.” She acknowledges, “If I weren’t a scientist”—trained in the iterative, painstaking process of evidence-based research—“that would just be frustrating to hear.”
Among the most fundamental questions about SARS-CoV-2 that bedevils epidemiologists is the virus’s reproductive number: how many people the average infected person infects. This is a key parameter used to model the spread of a disease, and to estimate what percentage of a population must be vaccinated (or have acquired immunity through infection) to reach “herd immunity”: the point at which even unvaccinated individuals in a population are protected. The most reliable way to calculate the reproductive number is to trace the spread of the virus from the date that “patient zero” was infected.

Megan Murray
Photograph by Stu Rosner
“Some researchers are estimating that the first case was in December 2019, but I really doubt that,” says Murray. “The infection could have been spreading for some time, as it did in many countries, more or less unnoticed. In places where people are young,” she points out, “many people are asymptomatic or have mild illness that they wouldn’t see a doctor about—or that a doctor wouldn’t pay much attention to if they didn’t know there was a novel virus spreading.”
Also complicating this picture are “super-spreader” events: the fact that under certain circumstances, one infected person can infect 90 or more others (as occurred during a February 2020 conference in Boston) while on other occasions, an infected individual infects no one. Super-spreader events amplify the “element of randomness” in viral spread, says Murray.
“It’s a little disappointing that…after a year and a half, we still don’t have a good estimate of how infectious the virus is, but we know enough,” she says. “I don’t think there’s much to be gained by estimating the exact proportion of people who would need to be vaccinated to reach herd immunity. These are useful concepts,” she continues, “but they’re not hard and fast rules. Too much stress has been put on a precision that isn’t possible”—and that may not matter much in places like the United States, where pockets of resistance to vaccination may make that goal unattainable in the near term.
What is known is that 50 to 60 percent of all transmission occurs when an asymptomatic person unwittingly infects others. The virus spreads when respiratory droplets or floating aerosolized particles, which can disperse like a gas, contact the mucous membranes of the mouth, nose, and eyes. The risk of transmission is highest indoors, particularly during vocalization and exercise, where suspended particles accumulate in poorly ventilated spaces (see harvardmag.com/pandemic-storm-20). Transmission through contact with infected surfaces is much less common; the early emphasis on hand-washing rather than mask-wearing was perhaps misplaced.
Those Infected
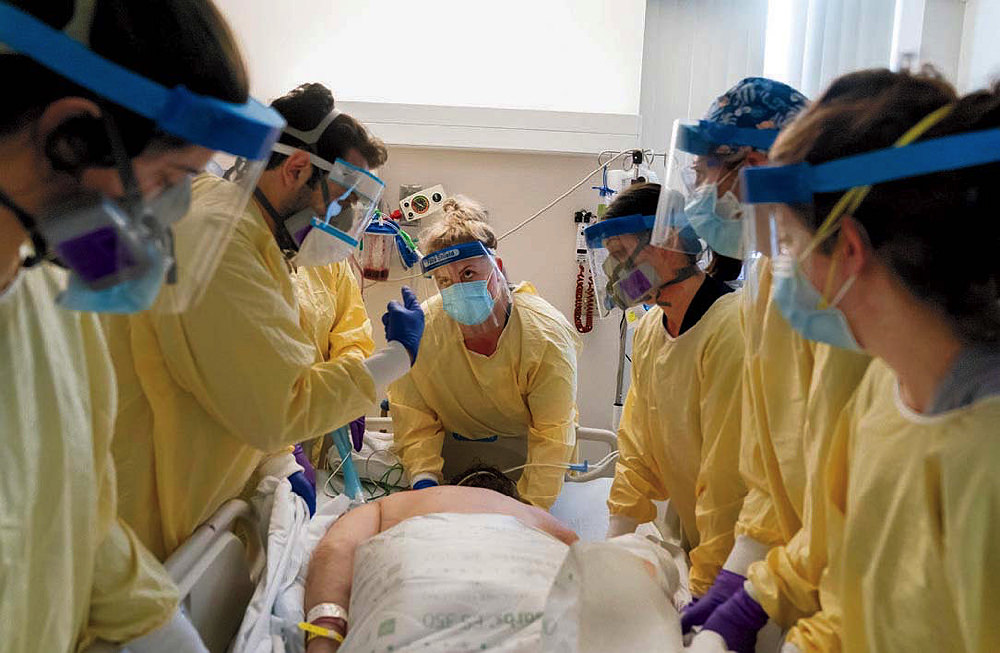
Wildly varied individual responses to infection are another characteristic of this pandemic. Imagine a large wedding at which 167 guests become infected with SARS-CoV-2. What are their outcomes? As many as 67 will never know they were infected. These asymptomatic people will later pass the virus on to others, driving future outbreaks. Of the remaining 100 guests, about 80 will develop mild to moderate symptoms not unlike a case of flu, but also potentially accompanied by difficulty breathing, and a loss of taste and smell (they, too, can pass the virus on to others before symptoms appear). About 15 to 20 guests will develop severe to critical disease, and two of them (2.3 on average) will die—although in nations with access to the most advanced care, recently developed treatments (described below) have improved their odds of survival.
Within the wedding party, people of any age may become infected, but the risk of severe illness is highest among the eldest. Risk rises steadily with age, doubling every five to six years, so that “when you get up into the 70s, 80s, and 90s,” says Murray, “then the risk is huge.” Also vulnerable are people with conditions such as obesity, diabetes, hypertension, chronic lung disease, or heart or kidney disease. Pregnant women who become infected are at greater risk of severe illness and death than other women of the same age, but are unlikely to pass the virus to an unborn child.
Surprisingly, seven to nine months after infection, even among the cohort of 80 guests who develop mild to moderate illness, a substantial portion will report lingering symptoms, including fatigue, loss of taste or smell, shortness of breath, and headaches. Some will experience a racing heart, trouble sleeping, inability to focus, fluctuating body temperature, depression, and anxiety. This so-called “long-Covid” is the subject of further research and will likely take years to understand (see harvardmag.com/long-covid-21).
Photograph by David Goldman/The Associated press
Children are at extremely low risk of severe disease. But very rarely, (at one in a hundred such weddings) a previously healthy child, most commonly between the ages of 3 and 12, will develop a condition called Multisystem Inflammatory Syndrome in Children (MIS-C), presenting with fever and shock weeks after infection with COVID-19 (often undiagnosed). MIS-C (and its adult counterpart, MIS-A) can damage the heart, lungs, kidneys, and other organs, and lead to death if not properly treated. Researchers at Harvard Medical School and two of its affiliated hospitals hypothesize that MIS-C occurs when viral particles that remain in the gut after infection pass into the bloodstream, initiating a hyperinflammatory response. Other researchers see similarities to toxic shock syndrome. But what causes the illness in some children and not others is unknown.
For the 15 to 20 wedding guests who suffer severe illness, potentially requiring hospitalization, the line between survival and death depends on the interaction between the virus and the immune system.
Treating a Multisystem Disease
“Compared to a year and half ago, when we knew nothing,” says BWH’s Lindsey Baden, “diagnosis and treatment of COVID-19 have come a long way. We can diagnose the disease through rapid nasal testing, we understand how it spreads, and we know how to protect ourselves while interacting with patients. Basic stuff, but it’s incredibly helpful.”

Lindsey Baden
Photograph by Stu Rosner
COVID-19 is “more than just an infection of the respiratory tract,” he explains. “It’s a multisystem disease.” The virus infects epithelium, a tissue found throughout the body. Flat and plate-like, epithelial cells line blood and lymphatic vessels, and protectively cover the organs, including the heart. A single layer of these cells forms the smooth walls of capillaries, acting as a membrane for transfer of circulating body fluids to and from tissues. In the lungs, epithelial cells line the alveolar air sacs, where the critical process of gas exchange takes place. When SARS CoV-2 attacks epithelium via a receptor protein called ACE2 (particularly abundant in the epithelial cells of the lung and small intestine), it disrupts alveolar function. Many patients exhibit low concentrations of blood oxygen when tested on arrival at the hospital.
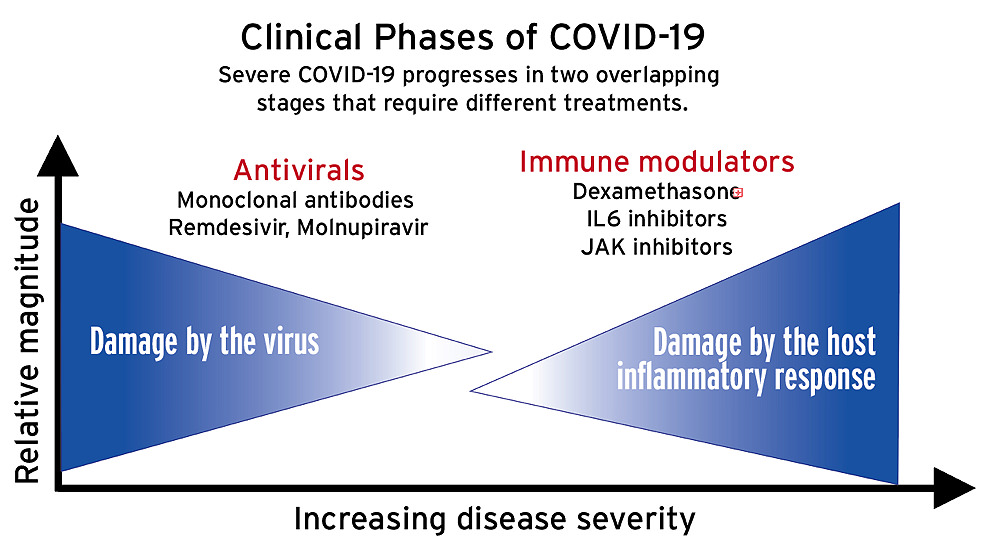
The disease proceeds in at least two overlapping phases (see illustration above). In the first, the virus itself causes damage in the lungs. In the second, a hyperinflammatory immune reaction, often referred to as a “cytokine storm,” causes damage to multiple organs, including the lungs and heart.*
Once a patient is hospitalized, predicting who will live or die has proved challenging. But there are clues.
Once a patient is hospitalized, predicting who will live or die has proved challenging. But there are clues. Patients at highest risk of pathogenic blood clotting, which probably develops when the body tries to repair injuries to the epithelium lining blood vessels, are those with high levels of blood biomarkers of inflammation, such as C-reactive protein (used to predict future cardiovascular events), and D-dimer, a protein fragment that is made when a blood clot dissolves. Anticoagulants help control this hallmark of severe COVID-19. Patients who successfully fight off COVID-19, post-hoc analysis reveals, generate a higher proportion of antibodies against the viral spike protein, the key structure that the virus uses to enter cells, than against other protein targets. (Vaccines train the immune system to focus on the spike). But predicting who will stage that optimally-targeted immune response remains an elusive goal.
Randomized, controlled trials have shown which treatments work—and which don’t. In the first phase of infection, “when viral replication is the driver of the disease,” says Baden, “antivirals like Remdesivir [that inhibit replication] and monoclonal antibodies [which block viral entry into cells by binding to the spike] developed from individuals who have recovered, target the virus very well.”
Treating the second, hyperinflammatory phase, during which the immune response itself is the cause of whole-body organ damage, involves dampening the immune system. “It’s a bit of a Goldilocks problem,” says Baden: “not too hot, not too cold.” Too much immune suppression invites infection; too little, and organ damage will ensue. Timing is crucial. If given too early, treatments like the steroid dexamethasone, an immune suppressant, will enhance viral replication. Patients who progress to the point of needing supplemental oxygen may be given other anti-inflammatories as well: tocilizumab, which inhibits the IL-6 inflammatory pathway (see harvardmag.com/spm-vs-inflammation-20) or the immunomodulators baricitinib or tofacitinib (rheumatoid arthritis drugs which work by reducing cytokine activity).

“We’re all a little different,” Baden points out, so treatments must be tailored to the patient, but throughout “the aim is to cool things off.” Some patients do become sicker. “But the numbers keep getting lower and lower, because at each of these stages, we prevent progression,” he notes. Recently, the federal government announced a plan to spend more than $3.2 billion developing new antiviral treatments that will target vulnerable steps in the SARS CoV-2 replication cycle, potentially adding to the treatment arsenal. Given that this coronavirus seems certain to become a chronic threat to humans, developing such therapies will be crucial.
Essential Work—and Inequity
Within the very first month of the epidemic’s outbreak in the United States, it became clear in hospitals like Baden’s that the typical COVID-19 patient was not, demographically speaking, just an unlucky wedding guest. The people most at risk of testing positive and requiring hospitalization were essential workers, people whose jobs did not allow them to work from home. This put their families and friends at risk, too.
Based on graphic by Healthy People 2030, U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion
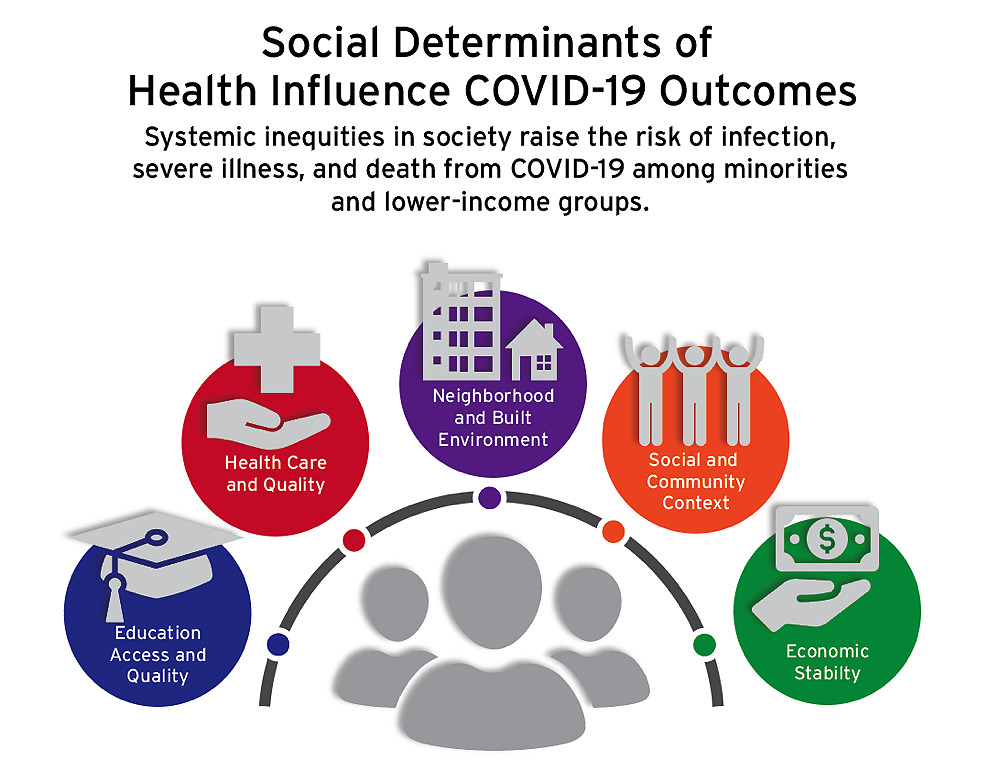
But even though such classic epidemiological factors could explain the overrepresentation of service and blue-collar workers among COVID-19 patients, it could not explain other disturbing data that began to emerge around the same time. Why were certain racial minorities approximately three times more likely to be hospitalized than whites—and more than twice as likely to die? These discrepancies could not be explained by genetic predispositions, because the observed health disparities varied from state to state. The inescapable conclusion was that socioeconomic determinants of health profoundly affect COVID-19 outcomes, including the risk of death. As Kolokotrones University Professor Paul Farmer put it in his recent book about Ebola, “the quaint notion that respiratory pathogens do not discriminate—because we all draw breath—is almost never true…” (see harvardmag.com/farmer-ebola-20). If lack of income or education leads to poorer nutrition, and is layered atop stress from working multiple jobs, while living near a polluting power plant, in overcrowded or substandard housing, for example, those social factors, which flow from public-policy decisions (the minimum wage, funds for public education, siting sources of pollution in poor neighborhoods) will ultimately affect health.
During pandemic-related remote learning, middle- and low-income student achievement began to dramatically lag that of high-income students…
The multiple causes and effects are mutually reinforcing: a study by Ackman professor of public economics Raj Chetty and colleagues at Opportunity Insights, a Harvard program he directs, showed that during pandemic-related remote learning, for example, middle- and low-income student achievement began to dramatically lag that of high-income students, who presumably had better access to technology, spaces conducive to learning, and support from parents whose employment allowed them to work from home. The pandemic has laid bare the effects of such disparities: black and Latino children were immediately overrepresented in cases of COVID-related Multisystem Inflammatory Syndrome in Children, a disease that didn’t exist before 2020.

Fixing the structural biases that lead to these outcomes is a long-term project, but health-care providers and federal agencies attempting to compensate by improving outreach in low-income communities have produced promising results. Vaccination programs, for example—deployed through pharmacies in minority neighborhoods, community vaccine centers, and mobile units, combined with trusted messengers—have been more successful than expected.
Vaccines and Variant Challenges
If resistant variants of the virus don’t defeat them, vaccines are the best hope to end the pandemic. “You don’t need to read a scientific paper” to know how well they are working, says Baden. By late June, Massachusetts was reporting fewer than 50 cases a day, he pointed out, down from 5,000 to 10,000 three to four months earlier. His hospital was caring for just three COVID patients, only one of them in intensive care. The anticipated fall seasonal surge among the unvaccinated notwithstanding, “What has happened in the last 100 days is just unbelievable, and it speaks volumes,” he continues. “Across the country, wherever there is a high uptake of vaccination, there is a dramatic drop in cases.”
Photograph by Mark Lennihan/The Associated Press
Baden himself continues to manage a small number of COVID-19 cases among immunocompromised patients. People with blood malignancies, or who are undergoing cancer treatment, for example, are sometimes unable to completely clear the virus. One such patient was a 45-year-old man with partial immune function. Sequencing revealed that the virus had rapidly evolved within him, developing specific mutations that foreshadowed identical changes in variants that later arose elsewhere around the world. One such mutation enables the virus to bind more efficiently to human cells. Another helps it avoid the immune response. “We’re watching convergent evolution,” Baden says, “as the virus in places like South Africa, Brazil, and the United Kingdom becomes better adapted to the human immune response through independent, synchronous mutations around the globe.”
While SARS CoV-2 is considered to be relatively stable compared to flu, the sheer number of cases globally (or in immunocompromised patients, the huge viral loads within a single person, evolving rapidly because of a partial immune response), has raised the odds that new, more transmissible variants will arise (see harvardmag.com/covid-mutations-21).
One that has been particularly well-studied is Alpha, formerly known as the U.K. variant, or B.1.1.7, which is estimated to be 50 percent more transmissible than the original strain used to create the vaccines. Data from the National Basketball Association, which successfully used extensive testing to reduce infections among its players (like many universities and some companies; see harvardmag.com/rapid-tests-21), indicates that this strain of SARS-CoV-2 achieves heightened transmissibility in two ways: it causes patients to shed more infectious particles at peak infection, and it causes a longer, 13-day infectious period (compared to the eight-day norm). Both changes increase the chances that an infected individual will infect others, thus raising the reproductive rate. The Delta variant that devastated India and is now sweeping the world is reportedly 60 percent more transmissible than Alpha, and twice as likely to result in hospitalization.
The rise of variants has raised questions about the ongoing efficacy of existing vaccines. Will they continue to work? Will they require boosters? Is there a test that could show when someone needs another shot? How well do the vaccines work in people with special conditions? All will take time to answer.
Reassuring news on that front came from Anthony Fauci, S.D. ’09, the director of the National Institute of Allergy and Infectious Diseases, during a White House briefing in early July. He reported that the Pfizer, Moderna, and Johnson & Johnson/Janssen (J&J) vaccines approved for emergency use in the United States showed similar antibody responses at six to eight months, and that all three cover Delta and the other common variants (studies of the Lambda and Delta Plus variants are continuing).

Dan Barouch
Photograph by Stu Rosner
“Some people have the impression that the J&J vaccine is not as potent as the mRNA vaccines” from Pfizer and Moderna, says Dan Barouch, who helped develop it. But a study of 20 healthy individuals given the J&J vaccine showed that antibody levels in recipients continue to improve over time, peaking more than two months after the single shot was given (and more than a month after efficacy was assessed at 29 days). These levels remained after eight months, with antibody and T-cell responses similar to those observed at one to two months. The Moderna and Pfizer vaccines induced a higher initial peak response, Barouch says, but then declined more steeply, so that all the vaccines show similar antibody responses at the six- to eight-month mark.
The study of the J&J vaccine also showed “improved responses to variants over time, which is something that I don’t think anyone would have predicted,” he continues, “and is definitely not seen with the other vaccines.” This maturation of the immune response “occurs even in the absence of further boosting.” As to longer-term effectiveness, a one-year study of a Zika vaccine that used the same adenovirus delivery mechanism as the J&J shot showed responses consistent with those elicited by the SARS CoV-2 vaccine, and durability of antibody levels for at least one year, when the work was suspended.
Equivalent research on the durability of mRNA vaccines is not yet available; the technology is too new. Baden, a deputy editor at The New England Journal of Medicine, points out that “We don’t even have a year of data to demonstrate efficacy.” (The Moderna clinical trial, the first of any vaccine, enrolled people in August and September of 2020.) “Yet there’s no shortage of pundits to say what we should, or shouldn’t be doing, and how long immunity lasts. It’s unsettling not being able to say that immunity is durable,” he acknowledges, “but we have to manage our discomfort with not knowing, and work with information being generated while we are in flight.”
Nonetheless, the efficacy of most vaccines wanes with time, so booster shots will likely be needed at some point—either when the level of immunity in the general population declines below an as-yet-to-be-determined threshold of protection, resulting in increasing numbers and clusters of vaccinated people getting infected; or a new variant emerges that more effectively escapes the current vaccine responses. “For now,” says Barouch, “I don’t think there is any need for boosters. But we do think that the virus has not fully explored its sequence space yet.” Researchers using computer models to simulate all the potential mutations that the SARS-CoV-2 virus could acquire believe the virus is still adapting, improving its ability to resist human immune defenses. Thus, he says, “We think it is possible that there could be variants in the future that are even more problematic than the ones that exist today.”
Researchers are using two approaches to establish correlates of protection (measurable immune factors that serve as a proxy for establishing immunity to disease) for the vaccines. The aim is to develop blood tests that can measure the effectiveness and durability of an individual’s immune response, thus establishing whether there is a need for boosters. Clinical trials are measuring the antibody levels that correspond with breakthrough infections (any SARS CoV-2 infection that occurs more than two weeks after vaccination; breakthroughs typically lead to mild disease). And preclinically, researchers are transferring purified antibodies into animals, challenging them with the virus, and measuring what antibody levels of each type are needed to establish immunity.
Neutralizing antibodies—those that block viral entry into cells by preemptively binding to and blocking spike proteins—are widely assumed to be a correlate of protection, says Barouch, but are not always linked to vaccine efficacy. Research on the mRNA vaccines, for example, show “substantial waning of neutralizing antibody responses,” after six months, he points out, but “protection against disease is maintained.” Similarly, the protection seen with the J&J vaccine in South Africa against the B.1.351 variant (Beta), remains “robust” even in patients with no neutralizing antibodies. A counter example comes from trials of the Novavax vaccine. That vaccine raised “astonishingly high levels of neutralizing antibodies,” Barouch points out, and “yet protection against the South Africa variant was only 49 percent.”
In the meantime, absent a blood test that would indicate how well a vaccine is working or the durability of its protection, preemptive research into boosting existing vaccines is under way. “My prediction is that, regardless of which vaccine you got initially,” Barouch says, “you will get a big boost of responses” with an extra shot. Multiple trials are testing optimal combinations and timing of doses from different manufacturers.
Vaccinating as many people as possible worldwide (and maintaining that protection) is both the humanitarian thing to do, Barouch says, and the smart thing: “[A]s long as the virus is surging anywhere”—raising the probability that resistant variants will emerge—“it puts everyone at risk.” During the next year, he is hopeful that the “combined manufacturing capacity of all the licensed vaccine producers will meet the global need.”
Preparing for the Next Pandemic
Epidemiologists had warned what to expect long before the first coronavirus case appeared. “Was I surprised that the pandemic was caused by a coronavirus?” asks Megan Murray. “After SARS in 2002”—she and a colleague created one of the first epidemiological models of that outbreak’s spread (see “The SARS Scare,” March-April 2007, page 48)—“we had MERS” in 2012. “Clearly coronaviruses had been shown to spill over from animal sources to humans and then rapidly adapt to human transmission. I know a lot of people were expecting flu [the pandemic of 1918, which killed an estimated 50 million people worldwide, was caused by a flu virus], but I never really had much of an opinion as to whether a big pandemic would be caused by flu or something else.”
“What everybody expected was that the next big pathogen would be respiratory…”
What Murray did expect—what “everybody expected,” she says—“was that the next big pathogen would be respiratory, because respiratory pathogens are transmissible in the context of modern life.” At no prior time in history have humans traveled farther or more frequently, nor lived so much of their lives indoors. “If this were the Middle Ages, we might have expected plague to spread due to poor sanitation and the possibility that people were in contact with fleas—or that a gastrointestinal virus that caused diarrheal disease, like cholera, would take off,” she continues. “Now, with lots of mobility, and closed and ventilated buildings, the respiratory pathogens” are the primary threat.
Clearly, as long as large numbers of people remain unvaccinated, by choice or not, the pandemic must be managed as a chronic threat. That implies continuing public-health measures that may include masks, limits on crowds, regular testing for infection, and contact tracing—as well as, inevitably, treatment for those who become ill.

Photograph by Chuck Nacke/Alamy Stock Photo
At the same time, global leaders must prepare for a successor pandemic, including one far worse than this one. Years of investment in research led to the creation of vaccine platforms, and to plans, albeit imperfect, to manufacture and distribute them globally. Could similar investments be made in public health? “Look at what happened with testing,” in the United States, says Barouch. “We had a head start, we knew it was coming, and we couldn’t figure out how to get people tested. We need a stronger public-health infrastructure, and better aligned political messaging.”
Knowing the nature of what to expect—a respiratory virus that thrives in the context of modern human behaviors—readiness will mean stockpiling nonexpired masks, working ventilators, and various levels of personal protective equipment for health-care workers. It will require that hospital systems develop strategies for swiftly expanding intensive-care capacity. Even scientists, already engaged in unprecedented collaboration, should develop even more sophisticated and deliberate ways of coordinating research and sharing results with each other and the public. To develop capacity and strategies for large-scale testing, federal and state public-health agencies will need to be strengthened. And government public-health announcements will need to convey a unified message, not just within nations, but globally. Making such investments sounds expensive. But nothing could be as costly as the loss of life and the trillions of dollars in economic damage that have already been inflicted by the COVID-19 crisis.
*Some models of the disease place a third, mainly pulmonary stage between these two stages, suggesting that hyperinflammation begins in the lungs when they try to repair themselves.