As you read these words, conditions in your body may feel peaceful. Savor that feeling, because it is a Herculean achievement on nature’s part. We owe this ability to sit reading quietly to a state of internal equilibrium—the technical term is homeostasis. This deceptive mantle of calm relies on an intricate choreography beneath the surface: picture air-traffic control at LaGuardia on a Friday evening. Under the skin, blood pulses, hormones circulate, microscopic proteins dart between cells. An incredibly complex web of signals transmits feelings of hunger and fullness, energy and fatigue, cuing the body to store energy or release it. We are oblivious to the hubbub within.
But in our bodies, as in the air-traffic control center, things can go wrong. A chronically overloaded flight schedule leads to chaos and, eventually, collapse. So, too, turning food into energy takes a toll. The body can metabolize a wide range of substances—fat, carbohydrate, protein—but this system begins to break down if chronically overloaded with excess calories that provide little nourishment. Our bodies’ equilibrium is remarkably resilient—but not endlessly so.
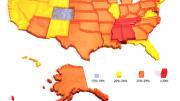
Public-health statistics reveal the repercussions of pushing the limits of this resilience with poor diet, too little exercise, and otherwise unhealthy lifestyles. Two-thirds of American adults are now overweight, according to the Centers for Disease Control (CDC); half of these are clinically obese. In the past 30 years, the prevalence of obesity has more than doubled. In 1985, there were only eight states where more than 10 percent of adult residents were obese; by 2001, there was not a single state with obesity prevalence below 15 percent (see the map opposite). “Collectively,” says Iacocca professor of medicine C. Ronald Kahn, “American adults gained two billion pounds during the 1990s.” (That’s roughly 10 pounds for each American adult, but an arresting figure nonetheless.)
Obesity, in turn, brings vulnerability to another debilitating metabolic dysfunction: diabetes. Someone with a body mass index above 40 (240 pounds for a person 5 feet, 5 inches tall; 295 pounds for a person 6 feet tall) is seven times as likely to develop the disease as someone of normal weight.
The CDC estimates that 18 million Americans have been diagnosed with diabetes, and almost 6 million more have it but have not been diagnosed. The cost of caring for diabetes and its complications accounts for one in every 12 dollars of healthcare spending in the United States. In fact, the health-related costs of obesity have surpassed those of smoking, notes Kahn, who from 2000 to 2007 directed the Harvard-affiliated Joslin Diabetes Center, which has 44 principal investigators and a research budget of $42 million this year. And, says Kahn, “the real impact of this hasn’t even been felt yet.” The CDC estimates that yet another 57 million Americans have prediabetes—elevated blood-sugar levels indicating the beginning of a breakdown in the body’s mechanisms for reining in blood sugar after a meal and for getting energy from food.
In the face of this looming public-health crisis, science is a source of hope. New discoveries—driven by research in genetics, cell metabolism, and the study of small molecules—are creating a vastly more nuanced understanding of the risk factors that underlie obesity and diabetes, and of how those factors operate in the body to bring about disease. With such knowledge comes the promise of new therapies, preventive measures, and perhaps even a cure.
BEYOND BLOOD SUGAR
Diabetes is a complex disease, but its medical definition is simple. In a healthy person, the body maintains blood glucose within a very tight range. (Commonly known as blood sugar, glucose is the body’s primary circulating energy source, produced by metabolism of ingested food or synthesis from energy stores.) To keep glucose in check, beta cells in the pancreas secrete insulin. This, in turn, signals cells in the fat tissue, muscles, and liver to take up excess glucose from the blood and reserve it for use later.
But in diabetics, both the ability to produce and the ability to respond to insulin are impaired, so blood sugar remains elevated. The diagnosis of diabetes rests entirely on glucose level, but the reasons the disease is dangerous lie downstream. Experts agree that with perfect control of blood sugar, it would be possible to prevent most or all of the disease’s complications. But monitoring blood sugar and injecting insulin, while life-saving, does not begin to approach the precision of the body’s own control. Over a period of years, repeated glucose spikes after meals damage the blood vessels, contributing to a host of complications: heart disease, hypertension, nerve damage, kidney failure. Diabetes is a leading cause of blindness as blood vessels in the retina are damaged; poor circulation in the feet leads to sores that won’t heal and, in a sadly high number of cases, amputation.
Some argue that developing better ways to monitor and manage blood sugar is the key to stemming the tide of diabetes-related healthcare costs, and certainly this is critical in the absence of a cure. But in the last decade, with the help of new research methods, scientists have been able to delve one layer deeper, fleshing out the insulin-glucose dichotomy to include contributions from other hormones, genes, organelles, and small molecules.
Consider the discovery of leptin, a hormone produced by adipose tissue, or fat. The hormone’s identification in 1994 by Rockefeller University molecular geneticist Jeffrey Friedman shook up existing notions of appetite, metabolism, and obesity. Up to that point, the scientific community had viewed fat as mere energy storage, excess calories socked away for later. Gradually, it came to view fat as an endocrine organ in its own right, secreting hormones and other molecular signals into the body. The discovery by Simmons professor of genetics and metabolism Gökhan Hotamisligil that fat emits inflammatory signals also informed this newly robust understanding of fat.

Gökhan Hotamisligil
Protein staining turns a cell’s endoplasmic reticula green. These organelles, responsible for folding newly made proteins, show up as a mesh-like network inside each cell. Endoplasmic reticulum stress, a state that results from caloric overload, is critical in the progression to diabetes.
Leptin has potent effects on hunger and physical activity. Mice that lack leptin due to genetic mutations weigh three times the normal amount and are lethargic; injected with the hormone (named after the Greek word leptos, meaning “thin”), the mice become more active, eat less, and lose weight.
Nor are these its only effects. Associate professor of medicine Christos Mantzoros has shown that in female athletes who are so lean they stop menstruating, leptin therapy will restore menstrual periods absent any weight gain or changes in diet—a finding that also has implications for the study and treatment of anorexia nervosa. Walker professor of medicine Jeffrey S. Flier, who is dean of Harvard Medical School (and in whose lab Mantzoros trained), discovered that although some obese humans have low leptin levels—and lose weight when given synthetic leptin—most obese people have abnormally high levels of the hormone. Flier soon realized that leptin resistance commonly accompanies obesity, akin to the insulin resistance that characterizes, and precedes, type 2 diabetes. In these people, evidently, leptin does not have its normal effect of inducing feelings of satisfaction and energy, so their bodies produce more and more, but to little effect, because the signals are scrambled.
This finding prompted questions about why and how leptin resistance arises. Flier was among those to note a structural similarity between leptin and cytokines, proteins used in intercellular communication. He suspected that a category of proteins known as SOCS—suppressors of cytokine signaling—acted on the cellular receptor system that handles leptin signaling. His findings confirmed the hypothesis: a protein called SOCS3 interferes with leptin action. Increase the SOCS3 level and mice show decreased response to leptin and don’t stop eating as soon; take away SOCS3, and mice are more sensitive to leptin.
But this signaling pathway, once identified, did not translate easily into a new therapy. Removing one substance from the body can leave receptors more sensitized to some other molecule that uses the same pathway; conversely, blocking the receptor may affect bodily processes far outside the intended consequences. In this case, it would seem desirable to design a drug that somehow immobilizes SOCS3 in the body and therefore pumps up leptin’s effects, were it not for another of the protein’s functions: limiting inflammation. Mice with SOCS3 genetically deleted die when injected with inflammatory cytokines. SOCS3, says Flier, is “one way the body protects itself from going into shock every five minutes.”
Even well-known biological mechanisms are often more complex, and more interconnected, than we ever imagined. Earlier diabetologists believed insulin’s relevant action was on the liver, signaling the organ to synthesize or store glucose. Then a series of experiments in mice, some of them in Ronald Kahn’s lab, showed that insulin also affects the brain, muscles, fat tissue, beta cells, and the endothelial cells of the blood-vessel walls. “It turns out,” says Kahn, “that all tissue has insulin receptors.” Such discoveries underscore the difficulty in devising a cure.
A newer line of research by Kahn illustrates that not all fat is created equal. Previous studies found an association between copious visceral fat, which accumulates around the internal organs, and health problems including insulin resistance and type 2 diabetes; subcutaneous fat, which resides just underneath the skin, is associated with improved insulin sensitivity and lower diabetes risk, particularly when that fat is located in the gluteofemoral region—the hips, thighs, and buttocks.
Kahn and colleagues used a fat-transplantation experiment in mice to test whether the operative factor was the fat’s location or, rather, some properties inherent in the fat. Their results, published in Cell Metabolism in May, imply that visceral fat and subcutaneous fat are fundamentally different: mice that had subcutaneous fat added to their abdominal cavities scored better on metabolic tests, while mice that had visceral fat added under their skin scored worse, indicating that the two types of fat tissue retained their original effects in a new location. The next challenge is to determine what makes subcutaneous fat salubrious and visceral fat harmful, and what factors—genetic or environmental—lead people to develop one type or the other.

Gökhan Hotamisligil
Comparing the adipose tissue of fat and thin individuals reveals major differences in structure and function, as shown in these slides of mouse tissue. Fat cells from a lean mouse (above left) are tightly packed and relatively uniform in shape and size; fat cells in an obese mouse (above right) have swollen up with stored lipids and become much larger. The purple dots between the cells are inflammatory cells and macrophages that cluster around dead and degenerated cells to engulf and digest them.
Even if we narrow the focus to consider only hormones (chemical messengers that travel the relatively long distance from one organ or type of tissue to another), the contributors to weight gain and diabetes go beyond insulin and leptin. Also involved are adiponectin, which acts similarly to leptin and is also secreted by fat; ghrelin, secreted by the stomach and pancreas to stimulate appetite; glucagon, secreted by the pancreas to tell the liver to release stored glucose for immediate use; and melanin concentrating hormone, which stimulates appetite and is generated in the brain.
At the same time that scientists are trying to understand the functions of known hormones, they are discovering entirely new ones. Just this year, researchers at the Harvard School of Public Health announced the discovery of a new class of hormones: lipokines. Unlike previously identified hormones, which are steroid- or protein-based, lipokines are made of lipids, or fats. Hotamisligil, postdoctoral fellow Haiming Cao, and colleagues used a novel technique to identify hundreds of fatty acids and pinpoint one that carried a message from mice’s fat cells to their muscles and livers, improving insulin sensitivity and blocking fat accumulation. Their work, published in Cell in September, also showed that obesity compromises the body’s ability to make this marvelous molecule.
Mapping these small-scale processes can seem like a game of whack-a-mole: understanding one small constituent part leads only to the realization of how much else isn’t understood. But the approach has already led to new therapies: several popular diabetes drugs act on signaling pathways—for instance, to stimulate insulin production, decrease glucose synthesis in the liver, and discourage fat storage. And teasing out each step has importance beyond finding drug targets; it helps distinguish primary changes from mere side effects in the progression to diabetes.
FROM HEALTH TO DISEASE
Obesity almost always precedes type 2 diabetes, but not everyone who becomes obese will go on to develop the disease. Does obesity cause diabetes, or is there some underlying factor that causes both conditions? So far, the answer appears to be “some of each.” Assistant professor of medicine Mary-Elizabeth Patti is among the researchers who are working on the road map that leads from health to disease, trying to figure out where the balance lies.
“By the time people get diabetes, there are many, many things that have changed,” says Patti, a researcher and endocrinologist at the Joslin. “Their glucose levels are high. Their insulin levels are lower than they should be, given the high glucose. Their lipids—circulating fats in their blood—are high.” Using medical imaging, scientists (the Joslin’s Alan M. Jacobson and Gail Musen among them) have revealed changes in both brain structure and brain function in diabetic patients, compared to those without the disease. The challenge is distinguishing cause from effect. Says Patti, “There are a lot of things that are already abnormal. Each of these things clouds the picture, so it’s hard to tell which are the primary changes.”
Setting out to learn what people with diabetes had in common from a genomic standpoint, Patti found impaired function in the expression of genes regulating mitochondria, the cellular powerhouses that convert glucose, lipids, and amino acids into ATP, the form of chemical energy that powers the body. This defect in one of the body’s most fundamental cellular components made sense. “If you don’t have as many mitochondria, or you don’t have normal function of mitochondria, you wouldn’t be able to oxidize or burn fuel, and that fuel would reside in cells and not be utilized well,” she says. But among people with high risk of developing diabetes because of obesity or family history, Patti did not find higher incidence of mitochondrial dysfunction, indicating that it is probably not the medium by which these risk factors coalesce into disease. Rather, she says, the results “suggest that mitochondrial dysfunction is an end result of everything that’s happened.”
Gökhan Hotamisligil believes he has a candidate for the culprit that tips a healthy human into a state of illness. He has zeroed in on a phenomenon called endoplasmic reticulum stress: a sort of energy overload, like an assembly line moving just slightly too fast for a worker to keep up. As the products move by, the worker can speed up a bit, but she tires faster, falls behind, and eventually gives up completely—at which point the products move by unattended and the whole operation collapses.
Like mitochondria, the endoplasmic reticula are organelles housed within cells, and they perform a function fundamental to life: folding newly made proteins and transporting them to the proper destinations. But the endoplasmic reticulum does not have an infinite capacity for increasing the rate at which it works: it “is highly sensitive to the energy and glucose level inside the cell,” says Hotamisligil, “and if demand is very high, it starts having trouble.” It emits an “SOS signal” in the form of enzymes called JNK, discovered by Hotamisligil’s lab in 2002, and IKK, identified around the same time by professor of medicine Steven E. Shoelson’s lab at the Joslin.
The medical world had long recognized that protein-folding problems played a critical part in cystic fibrosis and some neurodegenerative diseases, such as ALS (Lou Gehrig’s disease) and some forms of Alzheimer’s. But the idea of a link to diabetes was new. Now, Hotamisligil’s lab is working on “chemical chaperones,” compounds that they have already shown (in mice) to assist in protein folding and shore up the capacity of the endoplasmic reticulum, resulting in a reversal of diabetes. Pharmaceutical companies are hot on the trail of drugs that incorporate this technique, and also of JNK inhibitors that cancel the SOS signal and its effects downstream in the body.
THE INFLAMMATION CONNECTION
Elucidating the very specific roles of tiny proteins points to large-scale synergies in the body. Obesity typically accompanies a whole host of health problems: not just diabetes but often heart disease, hypertension, cirrhosis, impaired fertility, and even cancer. New research is revealing the ways in which whole systems and processes are interwoven—elegantly so in health, and vexatiously so in disease. Efforts to understand what exactly ties isolated health problems together into multifaceted disease have led scientists to focus on inflammation, a familiar physiological mechanism whose true import is only now becoming clear.
Many molecules implicated in type 2 diabetes—JNK, IKK, and SOCS3 among them—are components of the inflammatory signaling system, part of the body’s immune response. And these signals are activated by food intake.
Whenever we eat a meal, the body responds as if to an infection. In healthy people, this reaction, which accompanies the release of insulin in response to food, dies down after a time. The trouble seems to come when meals are so close together, or so inordinately large, that the body never gets a chance to recover from its inflamed state. “This ancient capacity of fat cells to produce an immune-like response is activated when they’re exposed to large amounts of energy,” says Hotamisligil. “The body starts perceiving excess amounts of energy as a foreign invader.”
While working in Hotamisligil’s lab, Kathryn Wellen, Ph.D. ’06, identified a new group of molecules called STAMPs that help fat cells cope with the onslaught of energy. Mice without these molecules developed metabolic problems (high blood sugar and lipids, insulin resistance, fatty liver, abdominal fat accumulation) when fed a normal diet; the hope is to harness STAMPs’ effects for use against the hazards of overeating in humans. Foods such as fruits and vegetables, herbs and spices, oily fish, and some nuts have natural anti-inflammatory properties, so a diet high in these foods also helps to mitigate this response.
Adipose tissue itself secretes pro-inflammatory molecules that are highly correlated with diabetes risk, independent of other factors. Professor of nutrition and epidemiology Frank B. Hu has found that obese people with high levels of interleukin-6—a potent inflammatory cytokine secreted by fat tissue—are more likely to develop diabetes than those with lower levels. On the other hand, people with high levels of adiponectin, an anti-inflammatory hormone also secreted by fat, enjoy a strong protective effect: people in the highest quintile for circulating adiponectin have a 90 percent reduced risk of getting diabetes. This effect held true in lean and obese subjects, whether active or sedentary, across all age groups. Because circulating levels of these substances are determined in part by genes, such findings help explain why some people are very resistant to developing diabetes, despite having multiple risk factors.

Map by Stephen Durke / Map data courtesy of S.H. Wild, G. Roglic, A. Green, R. Sicree, and H.King, &Ldquo;Global Prevalence of Diabetes Estimates for 2000 and Projections for 2030.&Rdquo; Diabetes Care 2004, 27:1047-1053
The United States’s car-centric culture and labor-saving appliances have saved Americans from having to walk anyplace or get even the minimal amount of physical activity involved in washing dishes by hand or wringing out laundry. Combined with the easy availability of cheap, highly processed, calorie-dense foods, the American lifestyle represents the “perfect storm” for diabetes. As this lifestyle continues to spread, it is a fair assumption that so will the American pattern of health problems. In rural China, for instance, diabetes incidence is less than 2 percent; in Hong Kong—with a genetically similar, but urbanized, population—the rate exceeds 10 percent.
Although diabetes incidence in the United States, Canada, and Europe is still relatively high, it is not because people of non-European descent have some sort of genetic protection. In fact, with similar lifestyles they are more likely to develop the disease. Diabetes incidence among non-Hispanic white American adults is 6.6 percent; among Asian Americans, it is 7.5 percent; among Hispanics, 10.4 percent; and among blacks, 11.8 percent.
Disparities persist even after controlling for factors such as diet and exercise. One factor: the threshold for developing diabetes varies among racial groups. For example, researchers with the Asian American Diabetes Initiative (https://aadi.joslin.harvard.edu) have found that for people of Asian ancestry, heightened diabetes risk begins at a body mass index of 23—well within the range the Centers for Disease Control considers normal. And this group is doubly vulnerable: people of Asian descent have higher genetic susceptibility to the disease’s complications, such as atherosclerosis.
If obesity and diabetes are a ticking time bomb in the United States, they pose an even larger threat in the developing world, where in many places the number of people with the disease is expected to double during the next two decades.
Particularly in obese individuals, adipose tissue contains clusters of macrophages, the immune-system cells that destroy and then digest invading pathogens. There are two types of macrophages: one that attacks viciously and kills alien microbes, and another that swoops in to repair the damage afterward, bringing about healing and tissue repair. The latter type is more plentiful in the fat tissue of lean people; obese people tend to have more of the former. Assistant professor of genetics and complex diseases Chih-Hao Lee is trying to parse cause and effect, and has found evidence for causality in both directions, in mouse models that presumably would translate to humans: inflammation makes an individual more prone to gaining weight, which makes the body’s baseline state more inflamed—a vicious cycle.
In a way, it makes perfect sense that inflammation and the immune response would be intimately linked to metabolism. Although the recognition of postprandial systemic inflammation is relatively recent, scientists have known for many years about another physiological state that triggers coordinated action. During acute infection, as the immune system fends off an invader, the body induces a temporary state of full-body insulin resistance, apparently because macrophages demand huge amounts of glucose. In a state of insulin resistance, the muscles, fat, and liver leave glucose circulating in the bloodstream instead of taking it up, and even release stored glucose to further bolster the immune response. Thus, the body gets the necessary power supply to counter the crisis of infection.
This phenomenon was taken for granted, perhaps because the articles documenting it were published so long ago, says Lee, that “when you do a PubMed search, you can’t even find them.” But recognition of connections between insulin action and metabolism on the one hand, and inflammation and the immune system on the other, has spurred renewed interest.
It has also spurred a rethinking of the medical dogma that type 1 and type 2 diabetes are entirely distinct. Type 1 has been—and still is—characterized as an autoimmune disease, while type 2 was considered altogether different. Although a type 2 patient’s body does not attack the beta cells in the pattern that defines type 1, scientists are beginning to recognize that the immune system plays a part in the development of type 2. And assistant professor of medicine Rohit Kulkarni, a researcher at the Joslin, has found that type 1’s pathogenesis is also not as simple as previously thought. Studying mice genetically predisposed to get type 1 diabetes, Kulkarni found that in those animals that eventually developed the disease, there was a breakdown of insulin signaling—the hallmark of type 2 diabetes—even before beta cells began to die off. It is also not lost on those who study the disease that the onset of type 1 diabetes sometimes follows an acute infection—that is, it follows the aforementioned full-body insulin resistance.
As the gap between the disease’s two variants shrinks, there is hope for new findings that shed light on both—and for therapies with dual applicability. “They are not the same,” says Hotamisligil. “But they are much more similar than we thought even five or six years ago.”