Editors’ note: Love it or hate it, exercise is a vital component of health. Harvard Magazine has explored exercise from its epidemiological impacts and its basic biology at the level of mitochondria, to its potent anti-inflammatory effects. Several other articles have covered the research of Lerner professor of biological sciences Daniel E. Lieberman, who brings an evolutionary and anthropological perspective to unpacking the paradox that humans are adapted to run (“Head to Toe,” January-February 2011, page 25), but have evolved to conserve energy, not expend it (“Born to Rest,” September-October 2016, page 9). In this feature, excerpted and adapted from chapter 10 of his new book Exercised: Why Something We Never Evolved to Do Is Healthy and Rewarding (Pantheon Books), he integrates diverse lines of evidence to explain what happens to the body during physical activity—and why it is healthy.
Everyone wants to live long, but no one wants to get old. So for centuries people have sought ways to slow aging and defer death. Not long ago, quacks would have tried to lure you to consume tobacco, mercury, or ground-up dog’s testicles to postpone your eternal rest; today’s peddlers of immortality hawk human growth hormone, melatonin, testosterone, mega-doses of vitamins, or alkaline food. For millennia, however, the most sensible advice has always included exercise. Just about everyone knows what countless studies confirm: regular physical activity slows the aging process and helps prolong life. I doubt anyone was astounded when Hippocrates wrote 2,500 years ago that “Eating alone will not make a man well; he must also take exercise.”
A mountain of evidence suggests that the Fountain of Youth runs with sweat. That sweat, moreover, needs to keep flowing as we age.
One of the most venerable long-term studies on how exercise affects aging is the Cooper Center Longitudinal Study in Dallas, Texas, started in 1970 by the man who coined the term “aerobics,” Dr. Kenneth Cooper. One of their analyses tracked more than 10,000 men and 3,000 women older than 35 years to test if people who exercised and were physically fit lived longer and healthier lives. They generally did. The more physically active women died at about one-third the rate of those who were unfit, and the fitter men had mortality rates about one-third to one-fourth lower than those who were least fit. Further, a subsample of those who were initially out of shape but started to exercise and increased their fitness halved their age-adjusted mortality rate compared to those who remained inactive and unfit. Because there is more to health than not being dead, Cooper Center researchers also tracked over the decades more than 18,000 healthy middle-aged individuals to see who got chronic health conditions such as diabetes and Alzheimer’s. Among both women and men, those who were more fit were about half as likely to suffer from chronic disease, and if they got sick did so at an older age. These and other such studies lend credence to the saying that “Men do not quit playing because they grow old; they grow old because they quit playing.”
It is understandable why many people are heedless or skeptical of the sorts of statistics I just cited. Everyone knows athletes who died tragically young and sedentary people who survived to old age. Further, those who avoid needless physical activity are simply doing what we evolved to do, especially as we age. Finally, even if “Exercise is Medicine,” how and why does physical activity affect how bodies age? Such questions behoove us to look beyond studies of just westerners and use evolutionary anthropological perspectives. They also require us to grapple with the age-old problem of why we get old in the first place. As it happens, humans age uniquely.
Old Age Over the Ages
Many memories of my grandparents involve them feeding me and my brother. Top marks go to my mother’s mother, whose specialty was breakfast. On weekends this first meal was a multi-course extravaganza that usually began with half a grapefruit, then hot cereal, then bagels with cream cheese and smoked salmon. Although less of a cook, my father’s mother never appeared without her signature sugar-free oatmeal cookies. My grandfathers got in the game, too. My mother’s father would drive around Brooklyn on Sunday mornings stopping at one deli for the best smoked salmon, another place for whitefish, and yet another for the perfect bagels. My father’s father always showed up with a giant salami and a tin of Dutch cocoa.

Illustration by Serge Bloch
In hindsight, my grandparents were doing in their own Brooklyn way what human grandparents—alone among species—have been doing for millions of years: feeding their grandchildren. This unique behavior is strongly linked to our exceptional longevity in which we typically live beyond the age at which we cease to reproduce. Similarly long post-reproductive lifespans are rare in the animal world. Chimpanzees, for example, seldom survive past the age of 50, soon after females go through menopause and after the age when males sire offspring. Kicking the bucket shortly after one has stopped producing and raising offspring makes sense from an evolutionary perspective. At this stage, organisms enter what biologist Peter Medawar termed the “shadow of natural selection.” Theoretically, once an individual falls into this dreaded shadow, it becomes biologically and evolutionarily obsolete because natural selection should no longer act to combat natural processes of aging.
Thankfully, elderly humans are anything but biologically obsolete. To understand how our extraordinary reproductive strategy rescued us, at least partially, from the coldhearted shadow of selection, consider that ape females raise just one dependent offspring at a time without much help. Chimpanzee mothers, for example, cannot give birth to babies faster than once every five to six years because they forage only enough food every day to sustain their caloric needs plus those of one hungry youngster. Not until her juvenile is old enough to be fully weaned and forage for itself can she muster enough calories to become fertile again. Human hunter-gatherers, in contrast, typically wean their offspring after three years and become pregnant again long before their little ones are able to feed or fend for themselves let alone stay out of danger. A typical hunter-gatherer mother, for example, might have a six-month-old infant, a four-year-old child, and an eight-year-old juvenile. Since she is usually capable of gathering only about 2,000 calories a day, she cannot get enough food to provide for her own substantial caloric needs, which exceed 2,000 calories, as well as the needs of her several offspring, none of whom is old enough to forage on its own. She needs help.
Among those who lend her a hand are middle-aged and elderly folks. Anthropologists have shown that grandmothers, grandfathers, aunts, uncles, and other older individuals in foraging populations from Australia to South America remain active throughout life, gathering and hunting more calories every day than they consume, which they provide to younger generations. This surplus food helps provide adequate calories to children, grandchildren, nieces, and nephews, and reduces how much work mothers have to do. Elderly hunter-gatherers also help younger generations by contributing knowledge, wisdom, and skills for about two to three decades beyond childbearing years. Contrary to the widespread assumption that hunter-gatherers die young, foragers who survive the precarious first few years of infancy are most likely to live to be 68 to 78 years old. That’s not far off from the life expectancy in the U.S., which is currently between 76 and 81.
The evidence that hunter-gatherers stay physically active for several decades after they stop having children is fundamental for understanding the nature of human aging. Most especially, our uniquely cooperative system of intergenerational cooperation and food-sharing postpones Medawar’s grim shadow. Instead of becoming obsolete, middle-aged and elderly hunter-gatherers bolster their reproductive success by provisioning children and grandchildren, doing childcare, processing food, passing on expertise, and otherwise helping younger generations. Once this novel cooperative strategy—the essence of the hunting and gathering way of life—started to emerge during the Stone Age, natural selection had the chance to select for longevity. According to this theory, hard-working and helpful grandparents who looked out for others and who were blessed with genes that favored a long life had more children and grandchildren, thus passing on those genes. Over time, humans were evidently selected to live longer to be generous, useful grandparents. One version of this idea is known as the Grandmother Hypothesis in recognition of the evidence that grandmothers play especially important roles.
In order to elucidate the links between exercise and aging, I propose a corollary to the Grandmother Hypothesis, which I call the Active Grandparent Hypothesis. According to this idea, human longevity was not only selected for but was also made possible by having to work hard during old age to help as many children, grandchildren, and other younger relatives as possible survive and thrive. That is, while there may have been selection for genes (as yet unidentified) that help humans live past the age of 50, there was also selection for genes that repair and maintain our bodies when we are physically active. As a result, many of the mechanisms that slow aging and extend life are turned on by physical activity, especially as we get older. Human health and longevity are thus extended both by and for physical activity.
Another way of stating the active grandparent hypothesis is that human longevity did not evolve to enable elderly humans to retire to Florida, sit by the pool, and ride around in golf carts. Instead, old age in the Stone Age meant plenty of walking, digging, carrying, and other forms of physical activity. In turn, natural selection favored older individuals whose bodies stimulated repair and maintenance mechanisms in response to the stresses caused by these activities. And since middle-aged and elderly humans never had the opportunity to retire and kick up their heels, there was never strong selection to turn on these mechanisms to the same degree without the stresses caused by physical activity. We thus evolved to toil by the sweat of our brows in middle and old age in order to live to be useful and helpful.
Let’s travel to northern Tanzania to visit the Hadza, one of the last hunter-gatherer tribes left on the planet, for a glimpse of what being over the hill in the Stone Age used to be like. A typical workday for a Hadza grandmother begins soon after dawn, tending to the fire and helping feed and care for her youngsters. A few hours later she along with other women in camp head out into the bush. They bring with them infants under two, which they carry in slings on their backs, and they are accompanied by children older than six or seven and sometimes an armed man or a couple of teenage boys to provide protection. Finding a good place to dig sometimes involves an hour-long trek. Once they find the vines that signal the presence of underground roots and tubers that are the staple of the Hadza diet, the women settle down to excavate. The main equipment is a digging stick, a thin piece of hardwood about the size of a cane whose end has been sharpened and hardened in a fire. Digging is arduous work because many tubers hide several feet deep under rocks that must be pried out, but the women chat as they work until mid-afternoon. Usually, everyone takes a break at midday for lunch. As with most Hadza cuisine, tubers are simply thrown on a fire and roasted for a few minutes and consumed there and then. After lunch comes yet more digging, and eventually the group heads home, carrying with them in slings whatever tubers were not yet consumed.
All Hadza women dig, but grandmothers dig more than mothers in part because they don’t have to nurse or spend as much time taking care of little ones. According to measurements by Kristen Hawkes, a professor at the University of Utah, and colleagues, a typical Hadza mother forages about four hours a day, but grandmothers forage five to six hours a day. On some days they dig less and spend more time collecting berries. And just as grandmothers head off every day to dig tubers and collect berries, grandfathers continue to hunt and to collect honey and baobab fruits, traveling just as far on most days as younger men. According to the anthropologist Frank Marlowe, “old men are the most likely to fall out of tall baobab trees to their deaths, since they continue to try to collect honey into old age.”
How many elderly Americans forage several hours a day, let alone climb trees and hunt animals on foot? We can, however, compare how much Americans and Hadza walk. A study of thousands found that the average twenty-first-century woman in the United States aged 18 to 40 walks 5,756 steps a day (about 2-3 miles), but this number declines precipitously with age, and by the time they are in their 70s, American women take roughly half as many steps. While Americans are half as active in their 70s as in their 40s, Hadza women walk twice as much per day as Americans with only modest declines as they age. In addition, research by David Raichlen from the University of Southern California and colleagues using heart-rate monitors showed that elderly Hadza women actually spent more of their day engaged in moderate to vigorous activity than younger women who were still having children. Imagine if elderly American women had to walk five miles a day to shop for their children and grandchildren, and instead of pulling items off the shelves, they had to dig for several hours in hard rocky soil for boxes of cereal, frozen peas, and fruit rollups.
Not surprisingly, hard work keeps elderly hunter-gatherers fit. One of the most reliable measures of age-related fitness is walking speed—a measure that correlates strongly with life expectancy. The average American woman under 50 walks about three feet per second (0.92 m/s) but slows down considerably to two feet per second (0.67 m/s) by her sixties. Thanks to an active lifestyle without retirement, there is no significant age-related decline in walking speed among Hadza women, whose average pace remains a brisk 3.6 feet per second (1.1 m/s) well into their 70s. Having struggled to keep up with elderly Hadza grandmas, I can attest they keep up a steady clip even when it is blistering hot. Older Hadza men also walk briskly. Studies of many hunter-gatherer groups attest that they attain higher levels of strength and fitness than typical post-industrial westerners and lose these capacities at a slower rate, remaining reasonably vigorous into old age. Debilitating muscle loss is not a problem among foragers.
The Active Grandparent Hypothesis raises a classic chicken-or-egg question. How much do humans live to old age so they can be active grandparents helping younger generations, or how much does their hard work cause them to live long lives in the first place? Is human longevity a result of physical activity or an adaptation to stay physically active? In addition, how did our hunter-gatherer ancestors deal with the inevitable selective shadow when they could no longer hunt and gather? Today, we have nursing homes, pensions, and government-funded health care to take care of senior citizens. Although elderly hunter-gatherers are afforded great respect, those who can’t walk long distances, dig tubers, collect honey, and schlepp stuff home presumably become burdens when food is limited. It follows that if humans were selected to live long after we stopped having babies, we were probably not selected to live those years in a state of chronic disability. From a Darwinian perspective, the best strategy is to live long and actively and then die fast when you become inactive. An even better strategy, however, would be to avoid any deterioration with age in the first place.
The Essence of Senescence
Sometimes when I look in the mirror I don’t recognize the grey-haired fellow with a receding hairline who stares back. Happily, I don’t yet feel as old as I look. Aging is inexorable, but senescence, the deterioration of function associated with advancing years, correlates much less strongly with age. Instead, senescence is also influenced strongly by environmental factors like diet, physical activity, or radiation, and thus can be slowed, sometimes prevented, and even partly reversed.
Aging is inexorable, but senescence, the deterioration of function associated with advancing years, is influenced strongly by environmental factors—and thus can be slowed or partly reversed.
At a mechanistic level, we senesce from a multitude of nasty processes. One worrying source of wear and tear arises from the chemical reactions that keep us alive. The oxygen we breathe generates energy in cells, but leaves behind unstable oxygen molecules. These reactive oxygen species (charmingly also called free radicals) steal electrons indiscriminately from other molecules, thereby “oxidizing” them. Just as oxidation causes metal to rust and apple flesh to brown, it damages cells throughout the body by zapping DNA, scarring the walls of arteries, inactivating enzymes, and mangling proteins. Paradoxically, the more oxygen we use, the more we generate reactive oxygen species, so theoretically vigorous physical activities that consume lots of oxygen should accelerate senescence.
A related driver of senescence is mitochondrial dysfunction. Mitochondria are the tiny power plants in cells that burn fuel with oxygen to generate energy (ATP), creating reactive oxygen species that, unchecked, cause self-inflicted damage. When mitochondria cease to function properly or dwindle in number they cause senescence and illness.
Another self-sabotaging reaction that results from being alive and using energy is browning, technically glycation. We brown when sugar and protein react with the help of heat. Glycation gives cooked foods like baked bread and roasted meat their dark, aromatic, tasty exteriors, but what’s good for cookies is bad for kidneys. These reactions can damage tissues and produce compounds (advanced glycation end products, AGEs) that stiffen blood vessels, wrinkle skin, harden the lenses in our eyes, clog up kidneys, and more. These and other kinds of damage then trigger inflammation.
In normal circumstances, the immune system stimulates inflammation to defend us from pathogens as well as self-inflicted damage caused by physical activity. In short bursts, inflammation is life-saving, but low levels of inflammation that last for months or years are pernicious because they slowly attack our bodies. Over time, the destructive effects of chronic, simmering inflammation accumulate in cells and tissues from head to toe, including in neurons in the brain, cartilage in joints, the walls of arteries, and insulin receptors in muscle and fat cells.
If oxidation, mitochondrial dysfunction, mutations, glycation, and inflammation were not enough, plenty of other processes also contribute to senescence by damaging and degrading cells. Over time, tiny molecules glue themselves to the DNA in cells. These so-called epigenetic (“on top of the genome”) modifications can affect which genes are expressed in particular cells. Because environmental factors like diet, stress, and exercise partly influence epigenetic modifications, the older we are, the more epigenetic modifications we accumulate. Most epigenetic modifications are harmless, but the more you have for a given age, the higher your risk of dying. Other forms of senescence include cells losing the ability to recycle damaged proteins; inadequately sensing and acquiring nutrients; and (less likely) being unable to divide because the little caps (telomeres) on the ends of chromosomes have become too short.
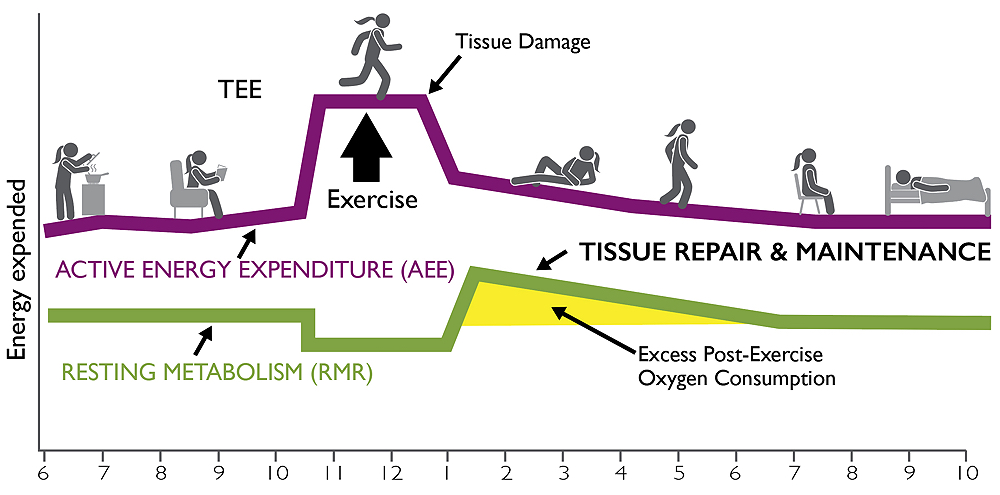
What Happens When We Exercise?
The graph breaks total energy expenditure (TEE) into two parts: active energy expenditure, and resting metabolism. Resting metabolism remains elevated for hours even after exercise ceases, burning additional calories in a phase known as excess post-exercise oxygen consumption (EPOC).
If this list of aging mechanisms alarms you, it should. Altogether, these mechanisms slowly wreak havoc. Plaque builds up in blood vessels, causing them to stiffen and clog. Receptors on cells become clogged. Muscle lose their mojo. Crud builds up around neurons and other key cells. Brain cells die. Membranes tear. Bones dwindle and crack. Tendons and ligaments fray. Our immune systems become less able to fight infections. Unless we repair these and many other forms of damage, our bodies break down like cars driven too many miles.
But there is hope. Aging and senescence are not inextricably linked because to varying degrees most of these destructive processes can be prevented or slowed and the damage they cause can be mended. Oxidation, for example, is halted by antioxidants, compounds that bind with reactive oxygen species, thus rendering them harmless. Some antioxidants like vitamins C and E come from food, but our bodies synthesize many other antioxidants in abundance. Similarly, mitochondria can be regenerated and some products of glycation can be repaired by enzymes that scavenge or break down these compounds. Inflammation can be turned off by anti-inflammatory proteins produced by white blood cells and muscles; telomeres can be lengthened; DNA can be repaired; and cells can be induced to restore or repair dozens of functions. Indeed, almost every cause of aging in almost every tissue (with a few glaring exceptions like hardening of the eyes’ lenses) can be countered, fixed, or prevented by one mechanism or another.
The body’s multitude of anti-aging mechanisms raises a conundrum: why don’t more humans—who we’ve already seen were selected to live longer than most animals—employ them earlier and more often to slow senescence and keep useful grandparents healthier for even longer?
Evolutionary biologists have been pondering this question for generations, and to cut to the chase, the best explanation by far is that natural selection becomes weaker as we age. On account of diseases, predators, harsh weather, and other cruelties of nature, older individuals inevitably become less common. As a result, natural selection acts less intensely on genes in elderly people that prolong life and promote repair. So even if middle- and old-aged people help out younger generations, thus postponing Medawar’s selective shadow, the older we get the less natural selection cares about fighting the accumulation of wear and tear that comes with age. Like it or not, the shadow eventually comes. But thankfully, its arrival and severity can be slowed and reduced by physical activity.
The Costly Repair Hypothesis
In 1966, a team of physiologists in Dallas, Texas, decided to compare the effects of sedentariness versus exercise on health by paying five healthy 20-year-olds to spend three weeks in bed, followed by an intensive eight-week exercise program. The bed rest was ruinous. When they were finally allowed to arise from their beds, the volunteers’ bodies resembled 40-year-olds by many metrics: they were fatter, had higher blood pressure, higher cholesterol levels, less muscle mass, and lower fitness. The eight ensuing weeks of exercise, however, not only reversed the deterioration but in some cases led to net improvements. To the lead researcher, Bengt Saltin, the take-home message was simple: “Humans were meant to move.” Time marches on, however, and to evaluate how aging affects the effects of inactivity, researchers had the bright idea of restudying the same five volunteers 30 years later.
Three decades of typical American lifestyles had not been kind to the original volunteers: they had each gained about 50 pounds, had higher blood pressure and weaker hearts, and were less fit and healthy in numerous ways. But they agreed to be studied once more as they tried to undo the consequences of 30 sedentary years with a six-month program of walking, cycling, and jogging. Fortunately, this second late-in-life exercise intervention helped the volunteers lose about 10 pounds, and, most astoundingly, largely reversed their decline in cardiovascular fitness. After six months of moderate exercise, the average volunteer’s blood pressure, resting heart rate, and cardiac output returned to his 20-year-old level. Many other studies confirm the anti-aging benefits of exercise. But few of them explain why.
Illustration by Serge Bloch
The most common explanation for why exercise slows and sometimes turns back the gradual slide toward poor health is that physical activity prevents or ameliorates bad things that accelerate senescence. Top of the list is fat. Exercise staves off and sometimes reverses the accumulation of excess fat, especially belly fat, a chief cause of inflammation and other problems. Exercise also lowers bloodstream levels of sugar, fat, and unhealthy cholesterol that slowly contribute to hardening of the arteries, damage proteins, and otherwise gum up the works. And as trials like the Dallas Bed Rest Study show, exercise also improves cardiovascular function, lowers levels of stress hormones, revs up metabolisms, strengthens bones, and more. Yet these and other salubrious effects of exercise explain only how but not why physical activity combats senescence. To understand why physical activity activates dozens of processes that maintain function and repair some of the damage that accumulates with age, we need to explore what I term the Costly Repair Hypothesis.
To introduce this idea, let’s follow (with her permission and the help of the image opposite) what happened to my wife when she included a hard gym workout as part of a typical Saturday. For the first few hours that day, my wife was either sedentary or did light physical activities. Then at 10:00 a.m. she went to the gym and did 45 minutes of vigorous cardio before a demanding 45-minute workout with weights. Afterward, she was not only tired but also slightly sore.
Importantly, my wife’s exercise session was not only calorically costly but also physiologically stressful. As she struggled to complete her cardio and weight workouts, her body’s “fight and flight” system released cortisol, epinephrine, and other stress-related hormones to speed up her heart and mobilize her energy reserves. As her muscles rapidly consumed calories, they pumped out waste compounds that compromised her cells’ functions, and her mitochondria leaked an abundance of harmful reactive oxygen species that damaged DNA and other molecules throughout the body. To add injury to insult, her hard-working muscles also developed microtears as she struggled with heavy weights. All in all, beyond causing discomfort, my wife’s strenuous workout generated some short-term damage.
If exercise is so destructive, why is it healthy? One explanation is that once she stopped exercising, my wife’s body reacted by repairing whatever harm she caused and, crucially, also repairing some of the damage that she had accumulated beforehand when she wasn’t exercising. As a result, she restored many tissues to their previous state. To deal with the tissue damage caused by her workout she mounted an initial inflammatory response followed by a later anti-inflammatory response. She also produced copious, powerful antioxidants to mop up the reactive oxygen species unleashed by her mitochondria. And she turned on a host of other processes to rid her cells of waste products, repair DNA mutations, damaged proteins, and epigenetic modifications, as well as mend cracks in her bones, replace and add mitochondria, and more.
Exercise is like scrubbing the kitchen floor so well after a spill that the whole floor ends up being cleaner. The modest stresses caused by exercise trigger a reparative response yielding a general benefit.
While exercise restores most structures (what biologists term homeostasis), in some cases it may create stability by making things even better than before (allostasis). For example, demanding physical activities can increase the strength of bones and muscles, increase cells’ abilities to uptake glucose from the blood, and both augment and replace mitochondria in muscles. In addition, repair mechanisms sometimes overshoot the damage induced by exercise, leading to a net benefit. It’s like scrubbing the kitchen floor so well after a spill that the whole floor ends up being cleaner. All in all, the modest physiological stresses caused by exercise trigger a reparative response yielding a general benefit, a phenomenon sometimes known as hormesis.
If you are entrepreneurial, hate exercise, or both, these beneficial responses may have ignited a lightbulb. Instead of going through the bother and discomfort of exercising, why not find some easier, preferably consumable way to turn on the same maintenance and repair mechanisms? Why not just take a pill? Without breaking a sweat, I can buy vitamins C and E and beta-carotene to boost my antioxidant levels, and purchase capsules loaded with turmeric, omega-3 fatty acids, and polyphenols that fight inflammation.
The problem is that dozens of studies have found that taking antioxidant pills is no substitute for physical activity to fight senescence. Three or four studies reported a modest benefit, but the rest found that antioxidants provided no benefit or even increased the risk of dying. To add insult to injury, additional studies suggest antioxidants may sometimes do more harm than good when combined with exercise. This head-turning conclusion followed from a groundbreaking 2009 experiment by Michael Ristow, a researcher in Zurich who studies aging and metabolism. His team asked 40 healthy young males with varied fitness levels to undergo four weeks of supervised exercise. Half the participants were given large doses of vitamins C and E, the other half received a placebo. Muscle biopsies taken before and after their exercise bouts showed that, as expected, physical activity induced plenty of oxidative stress, but those who took antioxidants incurred more oxidative damage because their bodies produced much lower levels of their own antioxidants. The antioxidant pills apparently suppressed the body’s normal anti-stress response, probably because oxidative damage from exercise itself is needed to trigger the body’s health-promoting antioxidative defense mechanisms.
Why is regular physical activity the best way to delay senescence and extend life?
Recall that according to the Costly Repair Hypothesis, organisms with restricted energy supplies (just about everyone until recently) must allocate limited calories toward either reproducing, moving, or taking care of their bodies, but natural selection ultimately cares only about reproduction. Consequently, the cold calculus of selection prefers us to spend as little energy as possible on costly maintenance and repair tasks. So while physical activities trigger cycles of damage and restoration, selection favors individuals who allocate enough but not too much energy to producing antioxidants, enlarging and repairing muscles, mending bones, and so on. The challenge is to maintain and repair any damage from physical activity just enough and in the right place and the right time.
Evolution’s stingy solution to this problem is to match capacity in response to demand. In this case, the demand is the stress caused by physical activity, especially reactive oxygen species and other damaging processes that stiffen arteries, mutate genes, and gunk up cells. The capacity is the ability to maintain, often through repair, a stable internal environment so we can adequately and effectively perform those functions needed for survival and reproduction. And, crucially, the maintenance and repair mechanisms activated by physical activity don’t cease to function as we age. Although some become less responsive, they keep on ticking, allowing physically active post-reproductive individuals to slow or delay senescence.
Just as our species never evolved to diet or cope with jetlag, we never evolved to counter many aging processes without physical activity, which activates repair responses.
Unfortunately, this marvelous system has one big flaw. Apparently, we never evolved to activate these maintenance and repair responses as effectively in the absence of regular physical activity. As we have seen, almost no one in the Stone Age, least of all grandparents, managed to avoid hours of walking, running, digging, climbing and other manual labors. Hunter-gatherers of all ages would have stimulated their body’s natural reparative mechanisms nearly every day in response to the demands posed by their way of life. So just as our species never evolved to diet or cope with jetlag, we never evolved to counter many aging processes to the same degree without physical activity.









